Abstract
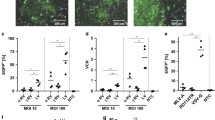
Currently, there is no reliable system for regulated gene expression and regulated gene knockdown in cells with finite lifespan. In this manuscript, we describe a vector system, consisting of a retrovirus for the delivery of rtTA, and a lentivirus for the delivery of either a transgene or a miR-shRNA for the modification of primary cells. Primary rat pulmonary microvascular endothelial cells (PMVEC) modified by these vectors for the inducible expression of Gaussia luciferase or DsRed Express demonstrated greater than 100-fold induction of the transgene expression with doxycycline. The system works reliably in both sequential and simultaneous infection modes, with about 95% of the sells selected with two antibiotics being inducible in each mode. The lentiviral vector for gene knockdown allows for the direct cloning of shRNA oligos using alpha-complementation, and for the monitoring of induction of RNA interference with fluorescent reporter, mCherry. The gene knockdown vector was validated by knocking down β-actin expression in PMVECs, with two of the four constructs showing 59 and 75% knockdown, respectively, compared to uninduced controls. The vectors described here were successfully used for the modification of various primary and established cell lines for regulated gene expression and regulated knockdown.




Similar content being viewed by others
References
Pohjoismaki JL, Wanrooij S, Hyvarinen AK et al (2006) Alterations to the expression level of mitochondrial transcription factor A, TFAM, modify the mode of mitochondrial DNA replication in cultured human cells. Nucleic Acids Res 34(20):5815–5828. doi:10.1093/nar/gkl703
Pastukh V, Shokolenko I, Wang B et al (2007) Human mitochondrial transcription factor A possesses multiple subcellular targeting signals. FEBS J 274(24):6488–6499. doi:10.1111/j.1742-4658.2007.06167.x
Sambrook J, Russel DW (2001) Molecular cloning. A laboratory manual. Cold Spring Harbor Laboratory Press, New York
King J, Hamil T, Creighton J et al (2004) Structural and functional characteristics of lung macro- and microvascular endothelial cell phenotypes. Microvasc Res 67(2):139–151. doi:10.1016/j.mvr.2003.11.006
Rappa G, Anzanello F, Alexeyev M et al (2007) Gamma-glutamylcysteine synthetase-based selection strategy for gene therapy of chronic granulomatous disease and graft-vs.-host disease. Eur J Haematol 78(5):440–448. doi:10.1111/j.1600-0609.2007.00833.x
Stegmeier F, Hu G, Rickles Rj et al (2005) A lentiviral microRNA-based system for single-copy polymerase II-regulated RNA interference in mammalian cells. Proc Natl Acad Sci USA 102(37):13212–13217. doi:10.1073/pnas.0506306102
Zufferey R, Nagy D, Mandel RJ et al (1997) Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol 15(9):871–875. doi:10.1038/nbt0997-871
Pear WS, Nolan GP, Scott ML et al (1993) Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA 90(18):8392–8396. doi:10.1073/pnas.90.18.8392
Wurdinger T, Badr C, Pike L et al (2008) A secreted luciferase for ex vivo monitoring of in vivo processes. Nat Methods 5(2):171–173. doi:10.1038/nmeth.1177
Loser P, Jennings GS, Strauss M et al (1998) Reactivation of the previously silenced cytomegalovirus major immediate-early promoter in the mouse liver: involvement of NFkappaB. J Virol 72(1):180–190
Brooks AR, Harkins RN, Wang P et al (2004) Transcriptional silencing is associated with extensive methylation of the CMV promoter following adenoviral gene delivery to muscle. J Gene Med 6(4):395–404. doi:10.1002/jgm.516
Paddison PJ, Caudy AA, Bernstein E et al (2002) Short hairpin RNAs (shRNAs) induce sequence-specific silencing in mammalian cells. Genes Dev 16(8):948–958. doi:10.1101/gad.981002
Pastukh V, Shokolenko IN, Wilson GL et al (2008) Mutations in the passenger polypeptide can affect its partitioning between mitochondria and cytoplasm: mutations can impair the mitochondrial import of DsRed. Mol Biol Rep 35(2):215–223. doi:10.1007/s11033-007-9073-7
Acknowledgments
This research was supported by grants PO1 HL06629907 and R21RR02396101
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alexeyev, M.F., Fayzulin, R., Shokolenko, I.N. et al. A retro-lentiviral system for doxycycline-inducible gene expression and gene knockdown in cells with limited proliferative capacity. Mol Biol Rep 37, 1987–1991 (2010). https://doi.org/10.1007/s11033-009-9647-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-009-9647-7