Abstract
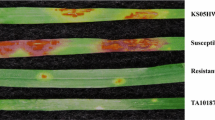
Spotted leaf 5 (spl5), a lesion mimic mutant, was first identified in rice (Oryza sativa L.) japonica cv. Norin8 in 1978. This mutant exhibits spontaneous disease-like lesions in the absence of any pathogens and resistance to rice blast and bacterial blight; however, the target gene has not yet been isolated. In the present study, we employed a map-based cloning strategy to finely map the spl5 gene. In an initial mapping with 100 F2 individuals (spl5/spl5) derived from a cross between the spl5 mutant and indica cv. 93-11, the spl5 gene was located in a 3.3-cM region on chromosome 7 using six simple sequence repeat (SSR) markers. In a high-resolution genetic mapping, two F2 populations with 3,149 individuals (spl5/spl5) were derived from two crosses between spl5 mutant and two indica cvs. 93-11 and Zhefu802 and six sequence-tagged site (STS) markers were newly developed. Finally, the spl5 gene was mapped to a region of 0.048 cM between two markers SSR7 and RM7121. One BAC/PAC contig map covering these markers’ loci and the spl5 gene was constructed through Pairwise BLAST analysis. Our bioinformatics analysis shows that the spl5 gene is located in the 80-kb region between two markers SSR7 and RM7121 with a high average ratio of physical to genetic distance (1.67 Mb/cM) and eighteen candidate genes. The analysis of these candidate genes indicates that the spl5 gene represents a novel class of regulators controlling cell death and resistance response in plants.



Similar content being viewed by others
References
Balague C, Lin B, Alcon C, Flottes G, Malmstrom S, Kohler C, Neuhaus G, Pelletier G, Gaymard F, Roby D (2003) HLM1, an essential signaling component in the hypersensitive response, is a member of the cyclic nucleotide-gated channel ion channel family. Plant Cell 15:365–379. doi:10.1105/tpc.006999
Buschges R, Hollricher K, Panstruga R, Simons G, Wolter M, Frijters A, van Daelen R, van der Lee T, Diergaarde P, Groenendijk J, Topsch S, Vos P, Salamini F, Schulze-Lefert P (1997) The barley Mlo gene: a novel control element of plant pathogen resistance. Cell 88:695–705. doi:10.1016/S0092-8674(00)81912-1
Das BK, Xia L, Palandjian L, Gozani O, Chyung Y, Reed R (1999) Characterization of a protein complex containing spliceosomal proteins SAPs 49, 130, 145, and 155. Mol Cell Biol 19:6796–6802
De Boer P, Vos HR, Faber AW, Vos JC, Raue HA (2006) Rrp5p, a trans-acting factor in yeast ribosome biogenesis, is an RNA-binding protein with a pronounced preference for U-rich sequences. RNA 12:263–271. doi:10.1261/rna.2257606
Dietrich RA, Delaney TP, Uknes SJ, Ward ER, Ryals JA, Dangl JL (1994) Arabidopsis mutants simulating disease resistance response. Cell 77:565–577. doi:10.1016/0092-8674(94)90218-6
Dietrich RA, Richberg MH, Schmidt R, Dean C, Dangl JL (1997) A novel zinc finger protein is encoded by the Arabidopsis LSD1 gene and functions as a negative regulator of plant cell death. Cell 88:685–694. doi:10.1016/S0092-8674(00)81911-X
Golas MM, Sander B, Will CL, Luhrmann R, Stark H (2003) Molecular architecture of the multiprotein splicing factor SF3b. Science 300:980–984. doi:10.1126/science.1084155
Gray J, Close PS, Briggs SP, Johal GS (1997) A novel suppressor of cell death in plants encoded by the Lls1 gene of maize. Cell 89:25–31. doi:10.1016/S0092-8674(00)80179-8
Hirochika H, Hirochika R (1993) Ty1-copia group retrotransposons as ubiquitous components of plant genomes. Jpn J Genet 68:35–46. doi:10.1266/jjg.68.35
Hoisington DA, Neuffer MG, Walbot V (1982) Disease lesion mimics in maize: I. Effect of genetic background, temperature, developmental age, and wounding on necrotic spot formation with Les1. Dev Biol 93:381–388. doi:10.1016/0012-1606(82)90125-7
Holton NJ, Goodwin TJD, Butler MI, Poulter RTM (2001) An active retrotransposon in Candida albicans. Nucleic Acids Res 29:4014–4024
Hu G, Yalpani N, Briggs SP, Johal GS (1998) A porphyrin pathway impairment is responsible for the phenotype of a dominant disease lesion mimic mutant of maize. Plant Cell 10:1095–1105
Hunt MD, Neuenschwander UH, Delaney TP, Weymann KB, Friedrich LB, Lawton KA, Steiner HY, Ryals JA (1996) Recent advances in systemic acquired resistance research—a review. Gene 179:89–95. doi:10.1016/S0378-1119(96)00429-5
Iwata N, Omura T, Satoh H (1978) Linkage studies in rice (Oryza sativa L.) on some mutants for physiological leaf spots. J Fac Agr Kyushu Univ 22:243–251
Johal G, Hulbert S, Briggs S (1995) Disease lesion mimic mutations of maize: a model for cell death in plants. Bioessays 17:685–692. doi:10.1002/bies.950170805
Kachroo P, Shanklin J, Shah J, Whittle EJ, Klessig DF (2001) A fatty acid desaturase modulates the activation of defense signaling pathways in plants. Proc Natl Acad Sci USA 98:9448–9453. doi:10.1073/pnas.151258398
Kramer A (1996) The structure and function of proteins involved in mammalian pre-mRNA splicing. Annu Rev Biochem 65:367–409. doi:10.1146/annurev.bi.65.070196.002055
Lee JY, Kwak JE, Moon J, Eom SH, Liong EC, Pedelacq JD, Berendzen J, Suh SW (2001) Crystal structure and functional analysis of the SurE protein identify a novel phosphatase family. Nat Struct Biol 8:789–794. doi:10.1038/nsb0901-789
Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K (1998) Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 10:1391–1406
Liu G, Wang L, Zhou Z, Leung H, Wang GL, He C (2004) Physical mapping of a rice lesion mimic gene, Spl1, to a 70-kb segment of rice chromosome 12. Mol Genet Genomics 272:108–115. doi:10.1007/s00438-004-1040-6
McCouch SR, Teytelman L, Xu Y, Lobos KB, Clare K, Walton M, Fu B, Maghirang R, Li Z, Xing Y, Zhang Q, Kono I, Yano M, Fjellstrom R, DeClerck G, Schneider D, Cartinhour S, Ware D, Stein L (2002) Development and mapping of 2240 new SSR markers for rice (Oryza sativa L.). DNA Res 9:199–207. doi:10.1093/dnares/9.6.199
Midoh N, Iwata M (1996) Cloning and characterization of a probenazole-inducible gene for an intracellular pathogenesis-related protein in rice. Plant Cell Physiol 37:9–18
Mizobuchi R, Hirabayashi H, Kaji R (2002) Isolation and characterization of rice lesion-mimic mutants with enhanced resistance to rice blast and bacterial blight. Plant Sci 163:345–353. doi:10.1016/S0168-9452(02)00134-6
Mori M, Tomita C, Sugimoto K, Hasegawa M, Hayashi N, Dubouzet JG, Ochiai H, Sekimoto H, Hirochika H, Kikuchi S (2007) Isolation and molecular characterization of a Spotted leaf 18 mutant by modified activation-tagging in rice. Plant Mol Biol 63:847–860. doi:10.1007/s11103-006-9130-y
Okamuro JK, Caster B, Villarroel R, Van Montagu M, Jofuku KD (1997) The AP2 domain of APETALA2 defines a large new family of DNA binding proteins in Arabidopsis. Proc Natl Acad Sci USA 94:7076–7081. doi:10.1073/pnas.94.13.7076
Panaud O, Chen X, McCouch SR (1996) Development of microsatellite markers and characterization of simple sequence length polymorphism (SSLP) in rice (Oryza sativa L.). Mol Gen Genet 252:597–607
Pandey GK, Grant JJ, Cheong YH, Kim BG, Li L, Luan S (2005) ABR1, an APETALA2-domain transcription factor that functions as a repressor of ABA response in Arabidopsis. Plant Physiol 139:1185–1193. doi:10.1104/pp.105.066324
Proudfoot M, Kuznetsova E, Brown G, Rao NN, Kitagawa M, Mori H, Savchenko A, Yakunin AF (2004) General enzymatic screens identify three new nucleotidases in Escherichia coli. Biochemical characterization of SurE, YfbR, and YjjG. J Biol Chem 279:54687–54694. doi:10.1074/jbc.M411023200
Ramirez SJ, Contreras FG, Gomez EMC (2005) Stationary phase in Escherichia coli. Rev Latinoam Microbiol 47:92–101
Schweizer P, Buchala A, Dudler R, Metraux JP (1998) Induced systemic resistance in wounded rice plants. Plant J 14:475–481. doi:10.1046/j.1365-313X.1998.00141.x
Takahashi A, Kawasaki T, Henmi K, Shi IK, Kodama O, Satoh H, Shimamoto K (1999) Lesion mimic mutants of rice with alterations in early signaling events of defense. Plant J 17:535–545. doi:10.1046/j.1365-313X.1999.00405.x
Tomiyama K (1963) Physiology and biochemistry of disease resistance of plants. Annu Rev Phytopathol 1:295–324. doi:10.1146/annurev.py.01.090163.001455
Venema J, Tollervey D (1996) RRP5 is required for formation of both 18S and 5.8S rRNA in yeast. EMBO J 15:5701–5714
Vos HR, Faber AW, de Gier MD, Vos JC, Raue HA (2004) Deletion of the three distal S1 motifs of Saccharomyces cerevisiae Rrp5p abolishes pre-rRNA processing at site A(2) without reducing the production of functional 40S subunits. Eukaryot Cell 3:1504–1512. doi:10.1128/EC.3.6.1504-1512.2004
Wang C, Wang H, Zhang J, Chen S (2008) A seed-specific AP2-domain transcription factor from soybean plays a certain role in regulation of seed germination. Sci China C Life Sci 51:336–345. doi:10.1007/s11427-008-0044-6
Wei Y, Zhang Z, Andersen CH, Schmelzer E, Gregersen PL, Collinge DB, Smedegaard-Petersen V, Thordal-Christensen H (1998) An epidermis/papilla-specific oxalate oxidase-like protein in the defence response of barley attacked by the powdery mildew fungus. Plant Mol Biol 36:101–112. doi:10.1023/A:1005955119326
Wolter M, Hollricher K, Salamini F, Schulze-Lefert P (1993) The mlo resistance alleles to powdery mildew infection in barley trigger a developmentally controlled defence mimic phenotype. Mol Gen Genet 239:122–128
Wu C, Bordeos A, Madamba MR, Baraoidan M, Ramos M, Wang GL, Leach JE, Leung H (2008) Rice lesion mimic mutants with enhanced resistance to diseases. Mol Genet Genomics 279:605–619. doi:10.1007/s00438-008-0337-2
Yamanouchi U, Yano M, Lin H, Ashikari M, Yamada K (2002) A rice spotted leaf gene, Spl7, encodes a heat stress transcription factor protein. Proc Natl Acad Sci USA 99:7530–7535. doi:10.1073/pnas.112209199
Yin Z, Chen J, Zeng L, Goh M, Leung H, Khush GS, Wang GL (2000) Characterizing rice lesion mimic mutants and identifying a mutant with broad-spectrum resistance to rice blast and bacterial blight. Mol Plant Microbe Interact 13:869–876. doi:10.1094/MPMI.2000.13.8.869
Zeng L, Yin Z, Chen J, Leung H, Wang GL (2002) Fine genetic mapping and physical delimitation of the lesion mimic gene Spl11 to a 160-kb DNA segment of the rice genome. Mol Genet Genomics 268:253–261. doi:10.1007/s00438-002-0743-9
Zeng DL, Qian Q, Dong GJ, Zhu XD, Dong FG, Teng S, Guo LB, Cao LY, Cheng SH, Xiong ZM (2003) Development of isogenic lines of morphological markers in indica rice. Acta Bot Sin 45:1116–1120
Zeng LR, Qu S, Bordeos A, Yang C, Baraoidan M, Yan H, Xie Q, Nahm BH, Leung H, Wang GL (2004) Spotted leaf11, a negative regulator of plant cell death and defense, encodes a U-box/armadillo repeat protein endowed with E3 ubiquitin ligase activity. Plant Cell 16:2795–2808. doi:10.1105/tpc.104.025171
Zhang QF, Shen BZ, Dai XK, Mei MH, Maroof MAS, Li ZB (1994) Using bulked extremes and recessive class to map genes for photoperiod-sensitive genic male sterility in rice. Proc Natl Acad Sci USA 91:8675–8679. doi:10.1073/pnas.91.18.8675
Acknowledgments
The authors thank the reviewers for helpful scientific comments. This work was supported by the National Natural Science Foundation of China (Grants no. 30370867 and 30771329), National Key Programs for Transgenic Crops (2008ZX08009-001, 003; 2009ZX08009-076B) and Zhejiang Normal University Innovative Research Team Program.
Author information
Authors and Affiliations
Corresponding authors
Additional information
X. Chen, J. Pan and J. Cheng contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, X., Pan, J., Cheng, J. et al. Fine genetic mapping and physical delimitation of the lesion mimic gene spotted leaf 5 (spl5) in rice (Oryza sativa L.). Mol Breeding 24, 387–395 (2009). https://doi.org/10.1007/s11032-009-9299-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11032-009-9299-2