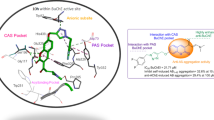
Abstract
Alzheimer’s disease (AD) is now ranked as the third leading cause of death after heart disease and cancer. There is no definite cure for AD due to the multi-factorial nature of the disease, hence, multi-target-directed ligands (MTDLs) have attracted lots of attention. In this work, focusing on the efficient cholinesterase inhibitory activity of tacrine, design and synthesis of novel arylisoxazole-tacrine analogues was developed. In vitro acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibition assay confirmed high potency of the title compounds. Among them, compounds 7l and 7b demonstrated high activity toward AChE and BChE with IC50 values of 0.050 and 0.039 μM, respectively. Both compounds showed very good self-induced Aβ aggregation and AChE-induced inhibitory activity (79.4 and 71.4% for compound 7l and 61.8 and 58.6% for compound 7b, respectively). Also, 7l showed good anti-BACE1 activity with IC50 value of 1.65 µM. The metal chelation test indicated the ability of compounds 7l and 7b to chelate biometals (Zn2+, Cu2+, and Fe2+). However, they showed no significant neuroprotectivity against Aβ-induced damage in PC12 cells. Evaluation of in vitro hepatotoxicity revealed comparable toxicity of compounds 7l and 7b with tacrine. In vivo studies by Morris water maze (MWM) task demonstrated that compound 7l significantly reversed scopolamine-induced memory deficit in rats. Finally, molecular docking studies of compounds 7l and 7b confirmed establishment of desired interactions with the AChE, BChE, and BACE1 active sites.
Graphic Abstract













Similar content being viewed by others
References
Patterson C (2018) World Alzheimer Report 2018—The State of the Art of Dementia Research: New Frontiers. Alzheimer’s Disease International (ADI), London
Deture MA, Dickson DW (2019) The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener 5:1–18. https://doi.org/10.1186/s13024-019-0333-5
https://alz-journals.onlinelibrary.wiley.com/doi/full/https://doi.org/10.1002/alz.12068
Piton M, Hirtz C, Desmetz C, Milhau J, Dominique-Lajoix A, Bennys K, Lehmann S, Gabelle A (2018) Alzheimer’s disease: advances in drug development. J Alzheimers Dis 65(1):3–13. https://doi.org/10.3233/JAD-180145
Oxford AE, Stewart ES, Rohn TT (2020) Clinical trials in Alzheimer's disease: A hurdle in the path of remedy. Int J Alzheimers Dis e5380346. https://doi.org/10.1155/2020/5380346.
Dos-Santos-Picanco LC, Ozela PF, de-Fatima-de-Brito M, Pinheiro AA, Padilha EC, Braga FS, de-Paula-da-Silva CHT, Dos-Santos CBR, Rosa JMC, da-Silva-Hage-Melim LI (2018) Alzheimer's disease: A review from the pathophysiology to diagnosis, new perspectives for pharmacological treatment. Curr Med Chem 25(26):3141-3159. https://doi.org/10.2174/0929867323666161213101126
Tobor TO (2019) On the etiopathogenesis and pathophysiology of Alzheimer’s disease: a comprehensive theoretical review. J Alzheimer’s Dis 68(2):417–437. https://doi.org/10.3233/JAD-181052
Teipel SJ, Fritz HC, Grothe MJ (2020) Alzheimer's disease neuroimaging initiative. Neuropathologic features associated with basal forebrain atrophy in Alzheimer disease. Neurology 95(10):1301–1311. https://doi.org/10.1212/WNL.0000000000010192.
Colautti J, Nagales K (2020) Tau and beta-amyloid in Alzheimer’s disease: Theories, treatments strategies, and future directions. Meducator 1(37):12–15. https://doi.org/10.15173/m.v1i37.2502
Castellani RJ, Plascencia-Villa G, Perry G (2019) The amyloid cascade and Alzheimer’s disease therapeutics: theory versus observation. Lab Invest 99:958–970. https://doi.org/10.1038/s41374-019-0231-z
Moussa-Pacha NM, Abdin SM, Omar HA, Alniss H, Al-Tel TH (2020) BACE1 inhibitors: current status and future directions in treating Alzheimer’s disease. Med Res Rev 40:339–384. https://doi.org/10.1002/med.21622
Bruni AC, Bernardi L, Gabelli C (2020) From beta amyloid to altered proteostasis in Alzheimer’s disease. Ageing Res Rev. https://doi.org/10.1016/j.arr.2020.101126
Cacabelos R (2020) Pharmacogenetic considerations when prescribing cholinesterase inhibitors for the treatment of Alzheimer’s disease. Expert Opin Drug Metab Toxicol 16(8):673–701. https://doi.org/10.1080/17425255.2020.1779700
Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, Delon MR (1982) Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science 215(4537):1237–1239. https://doi.org/10.1126/science.7058341
Agatonovic-Kustrin S, Kettle C, Morton DW (2018) A molecular approach in drug development for Alzheimer’s disease. Biomed Pharmacother 106:553–565. https://doi.org/10.1016/j.biopha.2018.06.147
Sharma K (2019) Cholinesterase inhibitors as Alzheimer’s therapeutics (Review). Mol Med Rep 20(2):1479–1487. https://doi.org/10.3892/mmr.2019.10374
Kabir MT, Uddin MS, Begum MM, Thangapandiyan S, Rahman MS, Aleya L, Mathew B, Ahmed M, Barreto GE, Ashraf GM (2019) Cholinesterase inhibitors for Alzheimer’s disease: multitargeting strategy based on anti-Alzheimer’s drugs repositioning. Curr Pharm Des 25(33):3519–3535. https://doi.org/10.2174/1381612825666191008103141
Martinez A, Castro A (2006) Novel cholinesterase inhibitors as future effective drugs for the treatment of Alzheimer’s disease. Expert Opin Invest Drugs 15(1):1–12. https://doi.org/10.1517/13543784.15.1.1
Ismaili L, Refouvelet B, Benchekroun M, Brogi S, Brindisi M, Gemma S, Campiani G, Filipic S, Agbaba D, Esteban G, Unzeta M, Nikolic K, Butini S, Marco-Contelles J (2017) Multitarget compounds bearing tacrine-and donepezil-like structural and functional motifs for the potential treatment of Alzheimer’s disease. Prog Neurobiol 151:4–34. https://doi.org/10.1016/j.pneurobio.2015.12.003
McEneny-King A, Osman W, Edginton AN, Rao PPN (2017) Cytochrome P450 binding studies of novel tacrine derivatives: predicting the risk of hepatotoxicity. Bioorg Med Chem Lett 27(11):2443–2449. https://doi.org/10.1016/j.bmcl.2017.04.006
Sameem B, Saeedi M, Mahdavi M, Shafiee A (2017) A review on tacrine-based scaffolds as multi-target drugs (MTDLs) for Alzheimer’s disease. Eur J Med Chem 128:332–345. https://doi.org/10.1016/j.ejmech.2016.10.060
Riazimontazer E, Sadeghpour H, Nadri H, Sakhteman A, Tüylü Küçükkılınç T, Miri R, Edraki N (2019) Design, synthesis and biological activity of novel tacrine-isatin Schiff base hybrid derivatives. Bioorg Chem 89:103006. https://doi.org/10.1016/j.bioorg.2019.103006
Makhaeva GF, Kovaleva NV, Boltneva NP, Lushchekina SV, Rudakova EV, Stupina TS, Terentiev AA, Serkov IV, Proshin AN, Radchenko EV, Palyulin VA, Bachurin SO, Richardson RJ (2020) Conjugates of tacrine and 1,2,4-thiadiazole derivatives as new potential multifunctional agents for Alzheimer’s disease treatment: synthesis, quantum-chemical characterization, molecular docking, and biological evaluation. Bioorg Chem 94:103387. https://doi.org/10.1016/j.bioorg.2019.103387
Korabecny J, Musilek K, Zemek F, Horova A, Holas O, Nepovimova E, Opletalova V, Hroudova J, Fisar Z, Jung YS, Kuca K (2011) Synthesis and in vitro evaluation of 7-methoxy-N-(pent-4-enyl)-1,2,3,4-tetrahydroacridin-9-amine-new tacrine derivate with cholinergic properties. Bioorg Med Chem Lett 21:6563–6566. https://doi.org/10.1016/j.bmcl.2011.08.042
Pan T, Xie S, Zhou Y, Hu J, Luo H, Li X, Huang L (2019) Dual functional cholinesterase and PDE4D inhibitors for the treatment of Alzheimer’s disease: design, synthesis and evaluation of tacrine-pyrazolo[3,4-b]pyridine hybrids. Bioorg Med Chem Lett 29(16):2150–2152. https://doi.org/10.1016/j.bmcl.2019.06.056
Najafi Z, Mahdavi M, Mahdavi M, Saeedi M, Karimpour-Razkenari E, Asatouri R, Vafadarnejad F, Homayouni-Moghadam F, Khanavi M, Sharifzadeh M, Akbarzadeh T (2017) Novel tacrine-1,2,3-triazole hybrids: In vitro, in vivo biological evaluation and docking study of cholinesterase inhibitors. Eur J Med Chem 125:1200–1212. https://doi.org/10.1016/j.ejmech.2016.11.008
Hu MK, Lu CF (2000) A facile synthesis of bis-tacrine isosteres. Tetrahedron Lett 41(11):1815–1818. https://doi.org/10.1016/S0040-4039(00)00036-8
Najafi Z, Mahdavi M, Saeedi M, Karimpour-Razkenari E, Edraki N, Sharifzadeh M, Khanavi M, Akbarzadeh T (2019) Novel tacrine-coumarin hybrids linked to 1,2,3-triazole as anti-Alzheimer’s compounds: In vitro and in vivo biological evaluation and docking study. Bioorg Chem 83:303–316. https://doi.org/10.1016/j.bioorg.2018.10.056
Saeedi M, Rastegari A, Hariri R, Mirfazli SS, Mahdavi M, Edraki N, Firuzi O, Akbarzadeh T (2020) Design and synthesis of novel arylisoxazole-chromenone carboxamides: investigation of biological activities associated with Alzheimer’s disease. Chem Biodivers 17(5):e1900746. https://doi.org/10.1002/cbdv.201900746
Vafadarnejad F, Mahdavi M, Karimpour-Razkenari E, Edraki N, Sameem B, Khanavi M, Saeedi M, Akbarzadeh T (2018) Design and synthesis of novel coumarin-pyridinium hybrids: In vitro cholinesterase inhibitory activity. Bioorg Chem 77:311–319. https://doi.org/10.1016/j.bioorg.2018.01.013
Najafi Z, Mahdavi M, Saeedi M, Sabourian R, Khanavi M, Safavi M, Tehrani MB, Shafiee A, Foroumadi A, Akbarzadeh T (2017) 1,2,3-Triazole-Isoxazole based acetylcholinesterase inhibitors: synthesis, biological evaluation and docking Study. Lett Drug Des Discov 14:58–65. https://doi.org/10.2174/1570180813666160628085515
Saeedi M, Safavi M, Allahabadi E, Rastegari A, Hariri R, Jafari S, Bukhari SNA, Mirfazli SS, Firuzi O, Edraki N, Mahdavi M, Akbarzadeh T (2020) Thieno[2,3-b]pyridine amines: synthesis and evaluation of tacrine analogs against biological activities related to Alzheimer’s disease. Arch Pharm 353:e2000101. https://doi.org/10.1002/ardp.202000101
Karimi-Askarani H, Iraji A, Rastegari A, Abbas-Bukhari SN, Firuzi O, Akbarzadeh T, Saeedi M (2020) Design and synthesis of multi-target directed 1,2,3-triazole-dimethylaminoacryloyl-chromenone derivatives with potential use in Alzheimer's disease. BMC Chem 14(1):pe64. https://doi.org/10.1186/s13065-020-00715-0.
Iraji A, Firuzi O, Khoshneviszadeh M, Tavakkoli M, Mahdavi M, Nadri H, Edraki N, Miri R (2017) Multifunctional iminochromene-2H-carboxamide derivatives containing different aminomethylene triazole with BACE1 inhibitory, neuroprotective and metal chelating properties targeting Alzheimer’s disease. Eur J Med Chem 141:690–702. https://doi.org/10.1016/j.ejmech.2017.09.057
Iraji A, Firuzi O, Khoshneviszadeh M, Nadri H, Edraki N, Miri R (2018) Synthesis and structure-activity relationship study of multi-target triazine derivatives as innovative candidates for treatment of Alzheimer’s disease. Bioorg Chem 77:223–235. https://doi.org/10.1016/j.bioorg.2018.01.017
Edraki N, Firuzi O, Fatahi Y, Mahdavi M, Asadi M, Emami S, Divsalar K, Miri R, Iraji A, Khoshneviszadeh M, Firoozpour L, Shafiee A, Foroumadi A (2015) N-(2-(Piperazin-1-yl)phenyl)arylamide derivatives as β-secretase (BACE1) inhibitors: simple synthesis by Ugi Four-component reaction and biological evaluation. Arch Pharm 348(5):330–337. https://doi.org/10.1002/ardp.201400322
Martorana A, Giacalone V, Bonsignore R, Pace A, Gentile C, Pibiri I, Buscemi S, Lauria A, Piccionello AP (2016) Heterocyclic scaffolds for the treatment of Alzheimer’s disease. Curr Pharm Des 22:3971–3995
Saeedi M, Mohtadi-Haghighi D, Mirfazli SS, Mahdavi M, Hariri R, Lotfian H, Edraki N, Iraji A, Firuzi O, Akbarzadeh T (2019) Design and synthesis of selective acetylcholinesterase inhibitors: Arylisoxazole-Phenylpiperazine derivatives. Chem Biodivers 16:e1800433. https://doi.org/10.1002/cbdv.201800433
Vafadarnejad F, Saeedi M, Mahdavi M, Rafinejad A, Karimpour-Razkenari E, Sameem B, Khanavi M, Akbarzadeh T (2017) Novel indole-isoxazole hybrids: synthesis and in vitro anti-cholinesterase activity. Lett Drug Des Discov 14:712–717. https://doi.org/10.2174/1570180813666161018124726
Vafadarnejad F, Karimpour-Razkenari E, Sameem B, Saeedi M, Firuzi O, Edraki N, Mahdavi M, Akbarzadeh T (2019) Novel N-benzylpyridinium moiety linked to arylisoxazole derivatives as selective butyrylcholinesterase inhibitors: Synthesis, biological evaluation, and docking study. Bioorg Chem 92:103192. https://doi.org/10.1016/j.bioorg.2019.103192
Ragab HM, Teleb M, Haidar HR, Gouda N (2019) Chlorinated tacrine analogs: Design, synthesis and biological evaluation of their anti-cholinesterase activity as potential treatment for Alzheimer’s disease. Bioorg Chem 86:557–568. https://doi.org/10.1016/j.bioorg.2019.02.033
Ragab HM, Ashour HMA, Galal A, Ghoneim AI, Haidar HR (2016) Synthesis and biological evaluation of some tacrine analogs: study of the effect of the chloro substituent on the acetylcholinesterase inhibitory activity. Monatsh Chem 147:539–552. https://doi.org/10.1007/s00706-015-1641-2
Keri RS, Quintanova C, Chaves S, Silva DF, Cardoso SM, Santos MA (2016) New tacrine hybrids with natural-based cysteine derivatives as multitargeted drugs for potential treatment of Alzheimer’s disease. Chem Biol Drug Des 87(1):101–111. https://doi.org/10.1111/cbdd.12633
Wilcken R, Zimmermann MO, Lange A, Joerger AC, Boeckler FM (2013) Principles and applications of halogen bonding in medicinal chemistry and chemical biology. J Med Chem 56(4):1363–1388. https://doi.org/10.1021/jm3012068
Iraji A, Khoshneviszadeh M, Firuzi O, Khoshneviszadeh M, Edraki N (2020) Novel small molecule therapeutic agents for Alzheimer disease: Focusing on BACE1 and multi-target directed ligands. Bioorg Chem 97:e103649. https://doi.org/10.1016/j.bioorg.2020.103649
Yazdani M, Edraki N, Badri R, Khoshneviszadeh M, Iraji A, Firuzi O (2020) 5,6-Diphenyl triazine-thio methyl triazole hybrid as a new Alzheimer’s disease modifying agents. Mol Divers 24:641–654. https://doi.org/10.1007/s11030-019-09970-3
Liao J, Nai Y, Feng L, Chen Y, Li M, Xu H (2020) Walnut oil prevents scopolamine-induced memory dysfunction in a mouse model. Molecules 25:pe1630. https://doi.org/10.3390/molecules25071630.
Vorhees CV, Williams MT (2006) Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1:848–858. https://doi.org/10.1038/nprot.2006.116
Brandeis R, Brandys Y, Yehuda S (1989) The use of the Morris Water Maze in the study of memory and learning. Int J Neurosci 48(1–2):29–69. https://doi.org/10.3109/00207458909002151
Acknowledgements
This work was supported by grants from the Research Council of Tehran University of Medical Sciences with project No. 98-01-33-41955.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of financial or personal interests that could have appeared to influence the content of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper is dedicated to our unique teacher in chemistry and medicinal chemistry (1937-2016)
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rastegari, A., Safavi, M., Vafadarnejad, F. et al. Synthesis and evaluation of novel arylisoxazoles linked to tacrine moiety: in vitro and in vivo biological activities against Alzheimer’s disease. Mol Divers 26, 409–428 (2022). https://doi.org/10.1007/s11030-021-10248-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-021-10248-w