Abstract
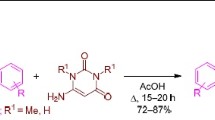
5-Arylidene-1-methyl-2-thiohydantoins undergo [3+2]-cycloaddition reaction with nitrile imines, generated in situ from hydrazonyl chlorides, at C=C and C=S dipolarophiles in the thiohydantoin moiety to afford thioxo-tetraazaspiro[4.4]nonenones and thia-tetraazaspiro[4.4]nonenones in moderate to good yields. The stereochemistry of these spiroheterocycles has been confirmed by X-ray diffraction studies.
Graphic abstract




Similar content being viewed by others
References
Ivasiv V, Albertini C, Gonçalves AE, Rossi M, Bolognesi ML (2019) Molecular hybridization as a tool for designing multitarget drug candidates for complex diseases. Curr Top Med Chem 19:1694–1711. https://doi.org/10.2174/1568026619666190619115735
Bérubé G (2016) An overview of molecular hybrids in drug discovery. Expert Opin Drug Discov 11:281–305. https://doi.org/10.1517/17460441.2016.1135125
Viegas-Junior C, Danuello A, da Silva BV, Barreiro EJ, Fraga CAM (2007) Molecular hybridization: a useful tool in the design of new drug prototypes. Curr Med Chem 14:1829–1852. https://doi.org/10.2174/092986707781058805
Sandhu S, Bansal Y, Silakari O, Bansal G (2014) Coumarin hybrids as novel therapeutic agents. Bioorg Med Chem 22:3806–3814. https://doi.org/10.1016/j.bmc.2014.05.032
Benabdallah M, Talhi O, Nouali F, Choukchou-Braham N, Bachari K, Silva A (2018) Advances in spirocyclic hybrids: chemistry and medicinal actions. Curr Med Chem 25:3748–3767. https://doi.org/10.2174/0929867325666180309124821
Hong X, Küçük HB, Maji MS, Yang YF, Rueping M, Houk KN (2014) Mechanism and selectivity of N-triflylphosphoramide catalyzed [3+2] cycloaddition between hydrazones and alkenes. J Am Chem Soc 136:13769–13780. https://doi.org/10.1021/ja506660c
Xu X, Zavalij PY, Doyle MP (2012) Synthesis of tetrahydropyridazines by a metal–carbene-directed enantioselective vinylogous N–H insertion/lewis acid-catalyzed diastereoselective mannich addition. Angew Chem Int Ed 51:9829–9833. https://doi.org/10.1002/anie.201203962
Kobayashi S, Shimizu H, Yamashita Y, Ishitani H, Kobayashi J (2002) Asymmetric intramolecular [3+2] cycloaddition reactions of acylhydrazones/olefins using a chiral zirconium catalyst. J Am Chem Soc 124:13678–13679. https://doi.org/10.1021/ja027681d
Saraswat P, Jeyabalan G, Hassan MZ, Rahman MU, Nyola NK (2016) Review of synthesis and various biological activities of spiro heterocyclic compounds comprising oxindole and pyrrolidine moities. Synth Commun 46:1643–1664. https://doi.org/10.1080/00397911.2016.1211704
Zheng YJ, Tice CM (2016) The utilization of spirocyclic scaffolds in novel drug discovery. Expert Opin Drug Discov 11:831–834. https://doi.org/10.1080/17460441.2016.1195367
Wang Y, Deng Y, Pang X, Yu J, Fan L, Chen Y, Zhao L (2017) Novel thiohydantoin analogues bearing the 1-hydroxyl-2,2,2-trifluoro-1-ethyl moiety as androgen receptor inhibitors for the potential treatment of castration resistant prostate cancer. RSC Adv 7:31866–31874. https://doi.org/10.1039/c7ra02142a
Bae YS, Choi S, Park JJ, Joo JH, Cui M, Cho H, Lee WJ, Lee SH (2016) Synthesis and biological evaluation of 3-substituted 5-benzylidene-1-methyl-2-thiohydantoins as potent NADPH oxidase (NOX) inhibitors. Bioorg Med Chem 24:4144–4151. https://doi.org/10.1016/j.bmc.2016.06.056
Majumdar P, Bathula C, Basu SM, Das SK, Agarwal R, Hati S, Singh A, Sen S, Das BB (2015) Design, synthesis and evaluation of thiohydantoin derivatives as potent topoisomerase I (Top1) inhibitors with anticancer activity. Eur J Med Chem 102:540–551. https://doi.org/10.1016/j.ejmech.2015.08.032
Chen Y, Su L, Yang X, Pan W, Fang H (2015) Enantioselective synthesis of 3,5-disubstituted thiohydantoins and hydantoins. Tetrahedron 71:9234–9239. https://doi.org/10.1016/j.tet.2015.10.041
Gazzeh H, Boudriga S, Askri M, Khatyr A, Knorr M, Strohmann C, Golz C, Rousselin Y, Kubicki MM (2016) Stoichiometry-controlled cycloaddition of nitrilimines with unsymmetrical exocyclic dienones: microwave-assisted synthesis of novel mono-and dispiropyrazoline derivatives. RSC Adv 6:49868–49875. https://doi.org/10.1039/c6ra09703k
Liu H, Jia H, Wang B, Xiao Y, Guo H (2017) Synthesis of spirobidihydropyrazole through double 1, 3-dipolar cycloaddition of nitrilimines with allenoates. Org Lett 19:4714–4717. https://doi.org/10.1021/acs.orglett.7b01961
Alizadeh A, Moafi L (2016) Convenient one-pot synthesis of spirooxindole derivatives containing a 1,3,4-thiadiazine scaffold. Synlett 27:1828–1831. https://doi.org/10.1055/s-0035-1561618
Hassaneen HM, Daboun HA, Abdelhadi HA, Abdel-Reheim NA (1995) Site selectivity and regiochemistry of nitrilimines. Cycloadditions to 1, 3-diphenyl-2-thiono-4-imidazolidinone and its 5-phenylmethylene derivatives. Phosphorus Sulfur Silicon Relat Elem 107:269–273. https://doi.org/10.1080/10426509508027942
Jakse R, Groselj U, Sorsak G (2007) Synthesis of thioaplysinopsin analogs derived from 5-dimethylaminomethylidene-2-thioxo-1,3-thiazol-4-ones. Heterocycles 73:743–750. https://doi.org/10.3987/COM-07-S(U)55
Yavari I, Khalili G (2010) A diastereoselective synthesis of phosphorylated dihydro-1H-pyrazoles from dialkyl phosphites, acetylenic esters, and hydrazonoyl chlorides. Synlett 12:1862–1864. https://doi.org/10.1055/s-0030-1258118
Yavari I, Nematpour M, Sodagar E (2015) Formation of spiro [indene-2,3′-pyrazole] derivatives from hydrazonyl chlorides and ninhydrin-malononitrile adduct. Monatsh Chem 146:2135–2138. https://doi.org/10.1007/s00706-015-1495-7
Yavari I, Nematpour M, Yavari S, Sadeghizadeh F (2012) Copper-catalyzed one-pot synthesis of tetrasubstituted pyrazoles from sulfonyl azides, terminal alkynes, and hydrazonoyl chlorides. Tetrahedron Lett 53:1889–1890. https://doi.org/10.1016/j.tetlet.2012.01.083
Yavari I, Taheri Z, Naeimabadi M, Bahemmat S, Halvagar MR (2018) A convenient synthesis of tetrasubstituted pyrazoles from nitrile imines and 2-(thioxothiazolidin-5-ylidene) acetates. Synlett 29:918–921. https://doi.org/10.1055/s-0036-1591921
Acknowledgements
We thank the Research Council of Tarbiat Modares University for supporting this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yavari, I., Taheri, Z., Sheikhi, S. et al. Synthesis of thia- and thioxo-tetraazaspiro[4.4]nonenones from nitrile imines and arylidenethiohydantoins. Mol Divers 25, 777–785 (2021). https://doi.org/10.1007/s11030-020-10056-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-020-10056-8