Abstract
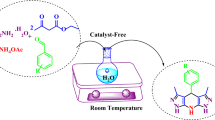
This research describes a simple and efficient one-pot synthetic approach for the preparation of tetrahydrodiazepine and dihydropyrazine (or dihydroquinoxaline) derivatives in high yields in the presence of a substoichiometric amount of ammonium chloride as a green accelerator on water at 50 °C within 1–3 h.
Graphical abstract





Similar content being viewed by others
References
Clark JH (1999) Green chemistry: challenges and opportunities. Green Chem 1:1
Sheldon RA, Arends I, Hanefeld U (2007) Green chemistry and catalysis. Wiley, New York
Anastas PT, Warner JC (2000) Green chemistry: theory and practice, vol 30. Oxford University Press, Oxford
Albini A, Protti S (2016) Paradigms in green chemistry and technology. Springer, Berlin
Gaich T, Baran PS (2010) Aiming for the ideal synthesis. J Organ Chem 75:4657
Wender P, Handy S, Wright D (1997) Towards the ideal synthesis: an everyday tool in the world of the chemical industry, syntheses are still some way from beingideal'. Chem Ind 765
Ugi I, Werner B, Dömling A (2003) The chemistry of isocyanides, their multicomponent reactions and their libraries. Molecules 8:53
Lubineau A, Augé J, Queneau Y (1994) Water-promoted organic reactions. Synthesis 1994:741
Jaiswal PK, Sharma V, Prikhodko J, Mashevskaya IV, Chaudhary S (2017) “On water” ultrasound-assisted one pot efficient synthesis of functionalized 2-oxo-benzo [1,4]oxazines: First application to the synthesis of anticancer indole alkaloid, Cephalandole A. Tetrahedron Lett 58:2077
Zeng L-Y, Liu T, Yang J, Yang Y, Cai C, Liu S-W (2017) “On-water” facile synthesis of novel pyrazolo [3,4-b] pyridinones possessing anti-influenza virus activity. ACS Comb Sci 19(7):437–446
Dömling A, Ugi I (2000) Multicomponent reactions with isocyanides. Angew Chem Int Ed 39:3168
Zhu J, Bienaymé H (2006) Multicomponent reactions. Wiley, New York
Ugi I, Dömling A, Hörl W (1994) Multicomponent reactions in organic chemistry. Endeavour 18:115
Nair V, Rajesh C, Vinod A et al (2003) Strategies for heterocyclic construction via novel multicomponent reactions based on isocyanides and nucleophilic carbenes. Acc Chem Res 36:899
Akritopoulou-Zanze I (2008) Isocyanide-based multicomponent reactions in drug discovery. Curr Opin Chem Biol 12:324
Pal R (2013) Recent progress of ammonium chloride as catalyst in organic synthesis. IOSR J Appl Chem 4:86
Bonne D, Dekhane M, Zhu J (2004) Ammonium chloride promoted ugi four-component, five-center reaction of α-substituted α-isocyano acetic acid: a strong solvent effect. Org Lett 6:4771
Dabiri M, Bahramnejad M, Baghbanzadeh M (2009) Ammonium salt catalyzed multicomponent transformation: simple route to functionalized spirochromenes and spiroacridines. Tetrahedron 65:9443
Larsen SD, Grieco PA (1985) Aza Diels-Alder reactions in aqueous solution: cyclocondensation of dienes with simple iminium salts generated under Mannich conditions. J Am Chem Soc 107:1768
Janvier P, Sun X, Bienaymé H, Zhu J (2002) Ammonium chloride-promoted four-component synthesis of pyrrolo [3,4-b]pyridin-5-one. J Am Chem Soc 124:2560
Shaabani A, Bazgir A, Teimouri F (2003) Ammonium chloride-catalyzed one-pot synthesis of 3, 4-dihydropyrimidin-2-(1H)-ones under solvent-free conditions. Tetrahedron Lett 44:857
Bonne D, Dekhane M, Zhu J (2005) Exploiting the dual reactivity of o-isocyanobenzamide: three-component synthesis of 4-imino-4 H-3, 1-benzoxazines. Org Lett 7:5285
Fayol A, Zhu J (2004) Synthesis of polysubstituted 4,5,6,7-tetrahydrofuro [2,3-c] pyridines by a novel multicomponent reaction. Org Lett 6:115
Foroughifar N, Mobinikhaledi A, Moghanian H, Mozafari R, Esfahani HR (2011) Ammonium chloride-catalyzed one-pot synthesis of tetrahydrobenzo [α] xanthen-11-one derivatives under solvent-free conditions. Synth Commun 41:2663
Mobinikhaledi A, Mosleh T, Foroughifar N (2015) Triethyl benzyl ammonium chloride (TEBAC) catalyzed solvent-free one-pot synthesis of pyrimido [4,5-d]pyrimidines. Res Chem Intermed 41:2985
Hussein E (2015) Ammonium chloride-catalyzed four-component sonochemical synthesis of novel hexahydroquinolines bearing a sulfonamide moiety. Russ J Org Chem 51:54
Ahumada G, Carrillo D, Manzur C, Fuentealba M, Roisnel T, Hamon J-R (2016) A facile access to new diazepines derivatives: Spectral characterization and crystal structures of 7-(thiophene-2-yl)-5-(trifluoromethyl)-2, 3-dihydro-1H-1, 4-diazepine and 2-thiophene-4-trifluoromethyl-1, 5-benzodiazepine. J Mol Struct 1125:781–787
Raboisson P, Marugán JJ, Schubert C et al (2005) Structure-based design, synthesis, and biological evaluation of novel 1,4-diazepines as HDM2 antagonists. Bioorg Med Chem Lett 15:1857
Fryer RI (2009) The chemistry of heterocyclic compounds, bicyclic diazepines: diazepines with an additional ring. Wiley, New York
Seitz LE, Suling WJ, Reynolds RC (2002) Synthesis and antimycobacterial activity of pyrazine and quinoxaline derivatives. J Med Chem 45:5604
Li J-Y, Cragoe E Jr, Lindemann B (1985) Structure-activity relationship of amiloride analogs as blockers of epithelial Na channels: I. Pyrazine-ring modifications. J Membr Biol 83:45
Boström J, Berggren K, Elebring T, Greasley PJ, Wilstermann M (2007) Scaffold hopping, synthesis and structure–activity relationships of 5, 6-diaryl-pyrazine-2-amide derivatives: a novel series of CB1 receptor antagonists. Bioorg Med Chem 15:4077
Fink M, Irwin P, Weinfeld RE, Schwartz MA, Canney AH (1976) Blood levels and electroencephalographic effects of diazepam and bromazepam. Clin Pharmacol Ther 20:184
Chowdary K, Rao YS (2003) Design and in vitro and in vivo evaluation of mucoadhesive microcapsules of glipizide for oral controlled release: a technical note. AAPS PharmSciTech 4:87
Pozarentzi M, Stephanidou-Stephanatou J, Tsoleridis CA (2002) An efficient method for the synthesis of 1,5-benzodiazepine derivatives under microwave irradiation without solvent. Tetrahedron Lett 43:1755
Shaabani A, Maleki A, Hajishaabanha F et al (2009) Novel syntheses of tetrahydrobenzodiazepines and dihydropyrazines via isocyanide-based multicomponent reactions of diamines. J Comb Chem 12:186
Shaabani A, Maleki A, Mofakham H, Moghimi-Rad J (2008) A novel one-pot pseudo-five-component synthesis of 4,5,6,7-tetrahydro-1 H-1,4-diazepine-5-carboxamide derivatives. J Organ Chem 73:3925
Neochoritis CG, Tsoleridis CA, Stephanidou-Stephanatou J, Kontogiorgis CA, Hadjipavlou-Litina DJ (2010) 1,5-Benzoxazepines vs 1,5-benzodiazepines. One-pot microwave-assisted synthesis and evaluation for antioxidant activity and lipid peroxidation inhibition. J Med Chem 53:8409
Shaabani A, Maleki A, Moghimi-Rad J (2007) A novel isocyanide-based three-component reaction: synthesis of highly substituted 1,6-dihydropyrazine-2, 3-dicarbonitrile derivatives. J Organ Chem 72:6309
Park Y-I, Son J-H, Kang J-S, Kim S-K, Lee J-H, Park J-W (2008) Synthesis and electroluminescence properties of novel deep blue emitting 6, 12-dihydro-diindeno [1, 2-b; 1′, 2′-e] pyrazine derivatives. Chem Commun 18:2143
Thakuria H, Das G (2006) One-pot efficient green synthesis of 1, 4-dihydro-quinoxaline-2, 3-dione derivatives. J Chem Sci 118:425
Li J, Liu Y, Li C, Jia X (2009) CAN-catalyzed syntheses of 3, 4-dihydroquinoxalin-2-amine derivatives based on isocyanides. Tetrahedron Lett 50:6502
Chari MA (2011) Amberlyst-15: an efficient and reusable catalyst for multi-component synthesis of 3, 4-dihydroquinoxalin-2-amine derivatives at room temperature. Tetrahedron Lett 52:6108
Shobha D, Chari MA, Mukkanti K, Kim SY (2012) Synthesis and anti-neuroinflammatory activity studies of substituted 3, 4-dihydroquinoxalin-2-amine derivatives. Tetrahedron Lett 53:2675
Kolla SR, Lee YR (2010) EDTA-catalyzed synthesis of 3,4-dihydroquinoxalin-2-amine derivatives by a three-component coupling of one-pot condensation reactions in an aqueous medium. Tetrahedron 66:8938
Maleki A (2012) Fe3O4/SiO2 nanoparticles: an efficient and magnetically recoverable nanocatalyst for the one-pot multicomponent synthesis of diazepines. Tetrahedron 68:7827
Shobha D, Chari MA, Sang L-C, Aldeyab SS, Mukkanti K, Vinu A (2011) Room-temperature multicomponent synthesis of 3,4-dihydroquinoxalin-2-amine derivatives using highly ordered 3D nanoporous aluminosilicate catalyst. Synlett 2011:1923
Shaabani A, Maleki A, Mofakham H (2008) Novel multicomponent one-pot synthesis of tetrahydro-1 H-1,5-benzodiazepine-2-carboxamide derivatives. J Comb Chem 10:595
Shaabani A, Soleimani E, Maleki A, Moghimi-Rad J (2009) A novel class of extended pi-conjugated systems: one-pot synthesis of bis-3-aminoimidazo [1,2-a] pyridines, pyrimidines and pyrazines. Mol Divers 13:269
Tracy DJ (1979) Catalytic effect of ammonium chloride on the synthesis of imidate esters. J Heterocycl Chem 16:1287
Shaabani A, Maleki A, Mofakham H, Khavasi HR (2008) Novel isocyanide-based three-component synthesis of 3, 4-dihydroquinoxalin-2-amine derivatives. J Comb Chem 10:323
Acknowledgements
We gratefully acknowledge financial support from the Iran National Science Foundation (INSF) and Research Council of Shahid Beheshti University.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shaabani, A., Sepahvand, H. & Ghasemi, S. Ammonium chloride-catalyzed green multicomponent synthesis of dihydropyrazine and tetrahydrodiazepine derivatives “on water”. Mol Divers 23, 585–592 (2019). https://doi.org/10.1007/s11030-018-9893-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-018-9893-5