Abstract
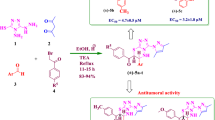
A series of new chiral 1,3,4-thiadiazole-based bis-sulfonamides 4a–4w and tri-sulfonamide analogue 5 was synthesized and evaluated as anti-HIV agents. The reaction of chiral amino acids 1 with sulfonyl chlorides 2, followed by subsequent reaction of resultant N-protected amino acids 2a–2f with thiosemicarbazide in the presence of excess phosphorous oxychloride afforded N-(1-(5-amino-1,3,4-thiadiazol-2-yl)alkyl)-4-arylsulfonamides 3a–3f. Treatment of 2a–2f with substituted sulfonyl chlorides in portions furnished the target bis-sulfonamide analogues 4a–4w in good yields, together with the unexpected 5. The new compounds were assayed against HIV-1 and HIV-2 in MT-4 cells. Compounds 4s were the most active in inhibiting HIV-1 with IC50 = 9.5 μM (SI = 6.6), suggesting to be a new lead in the development of an antiviral agent. Interestingly, compound 5 exhibited significant cytotoxicity of > 4.09 μM and could be a promising antiproliferative agent.



Similar content being viewed by others
References
Li Y, Geng J, Liu Y, Yu S, Zhao G (2013) Thiadiazole a promising structure in medicinal chemistry. ChemMedChem 8:27–41. https://doi.org/10.1002/cmdc.201200355
Hu Y, Li C-Y, Wang X-M, Yang Y-H, Zhu H-L (2014) 1,3,4-Thiadiazole: synthesis, reactions, and applications in medicinal, agricultural, and materials chemistry. Chem Rev 114:5572–5610. https://doi.org/10.1021/cr400131u
Jain AK, Sharma S, Vaidya A, Ravichandran V, Agrawal RK (2013) 1,3,4-Thiadiazole and its derivatives: a review on recent progress in biological activities. Chem Biol Drug Des 81:557–576. https://doi.org/10.1111/cbdd.12125
Ezabadi IR, Camoutsis C, Zoumpoulakis P, Geronikaki A, Soković M, Glamocilija J, Cirić A (2008) Sulfonamide-1,2,4-triazole derivatives as antifungal and antibacterial agents: synthesis, biological evaluation, lipophilicity, and conformational studies. Bioorg Med Chem 16:1150–1161. https://doi.org/10.1016/j.bmc.2007.10.082
Kumura K, Wakiyama Y, Ueda K, Umemura E, Watanabe Yamamoto M, Yoshida T, Ajito K (2018) Synthesis and antibacterial activity of novel lincomycin derivatives. III. Optimization of a phenyl thiadiazole moiety. J Antibiot 71:104–112. https://doi.org/10.1038/ja.2017.59
Chen C, Song B, Yang S, Xu G, Bhadury PS, Jin L, Hu D, Li Q, Liu F, Xue W, Lu P, Chen Z (2007) Synthesis and antifungal activities of 5-(3,4,5-trimethoxyphenyl)-2-sulfonyl-1,3,4-thiadiazole and 5-(3,4,5-trimethoxyphenyl)-2-sulfonyl-1,3,4-oxadiazole derivatives. Bioorg Med Chem 15:3981–3989. https://doi.org/10.1016/j.bmc.2007.04.014
Liu X-H, Shi Y-X, Ma Y, Zhang C-Y, Dong W-L, Pan L, Wang B-L, Li B-J, Li Z-M (2009) Synthesis, antifungal activities and 3D-QSAR study of N-(5-substituted-1,3,4-thiadiazol-2-yl)cyclopropanecarboxamides. Eur J Med Chem 44:2782–2786. https://doi.org/10.1016/j.ejmech.2009.01.012
Klip NT, Capan G, Gursoy A, Uzun M, Satana D (2010) Synthesis, structure, and antifungal evaluation of some novel 1,2,4-triazolylmercaptoacetylthiosemicarbazide and 1,2,4-triazolylmercaptomethyl-1,3,4-thiadiazole analogs. J Enz Inhib Med Chem 25:126–131. https://doi.org/10.3109/14756360903040439
Kadi AA, Al-Abdullah ES, Shehata IA, Habib EE, Ibrahim TM, El-Emam AA (2010) Synthesis, antimicrobial and anti-inflammatory activities of novel 5-(1-adamantyl)-1,3,4-thiadiazole derivatives. Eur J Med Chem 45:5006–5011. https://doi.org/10.1016/j.ejmech.2010.08.007
Tozkoparan B, Aytac SP, Gursoy S, Gunal S, Aktay G (2012) Novel 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazole derivatives as dual analgesic/anti-inflammatory and antimicrobial agents. Lett Drug Des Discov 9:204–212. https://doi.org/10.2174/157018012799079626
Bhat AR, Tazeem Azam A, Choi I, Athar F (2011) 3-(1,3,4-Thiadiazole-2-yl)quinoline derivatives: synthesis, characterization and antimicrobial activity. Eur J Med Chem 46:3158–3166. https://doi.org/10.1016/j.ejmech.2011.04.013
Bansode S, Kamble R (2011) Synthesis of novel 2-(3′-aryl-sydnon-4′-ylidene)-5′-substituted-[1,3,4]-thiadiazolylamines and [1,3,4]-thiadiazol-2′-yl-3-oxo-[1,2,4]-triazoles as antimicrobial agents. Med Chem Res 21:867–873. https://doi.org/10.1007/s00044-011-9596-2
Onkol T, Doğruer DS, Uzun L, Adak S, Ozkan S, Sahin MF (2008) Synthesis and antimicrobial activity of new 1,2,4-triazole and 1,3,4-thiadiazole derivatives. J Enzyme Inhib Med Chem 23:277–284. https://doi.org/10.1080/14756360701408697
Talath S, Gadad AK (2006) Synthesis, antibacterial and antitubercular activities of some 7-[4-(5-amino-[1,3,4]thiadiazole-2-sulfonyl)-piperazin-1-yl]fluoroquinolonic derivatives. Eur J Med Chem 41:918–924. https://doi.org/10.1016/j.ejmech.2006.03.027
Foroumadi A, Soltani F, Jabini R, Moshafi MH, Rasnani FM (2004) Antituberculosis agents X. Synthesis and evaluation of in vitro antituberculosis activity of 2-(5-nitro-2-furyl)- and 2-(1-methyl-5-nitro-lH-imidazol-2-yl)-1,3,4-thiadiazole derivatives. Arch Pharm Res 27:502–506. https://doi.org/10.1007/BF02980122
Chitra S, Paul N, Muthusubramanian S, Manisankar P, Yogeeswari P, Sriram D (2011) Synthesis of 3-heteroarylthioquinoline derivatives and their in vitro antituberculosis and cytotoxicity studies. Eur J Med Chem 46:4897–4903. https://doi.org/10.1016/j.ejmech.2011.07.046
Kumar D, Kumar NM, Chang K-H, Shah K (2010) Synthesis and anticancer activity of 5-(3-indolyl)-1,3,4-thiadiazoles. Eur J Med Chem 45:4664–4668. https://doi.org/10.1016/j.ejmech.2010.07.023
Bhole RP, Bhusari KP (2010) Synthesis and antitumor activity of (4-hydroxyphenyl)[5-substituted alkyl/aryl)-2-thioxo-1,3,4-thiadiazol-3-yl]methanone and [(3,4-disubstituted)-1,3-thiazol-2ylidene]-4-hydroxybenzohyd-razide. Med Chem Res 20:695–704. https://doi.org/10.1007/s00044-010-9371-9
Chou J, Lai S, Pan S, Jow G, Chern J, Guh J (2003) Investigation of anticancer mechanism of thiadiazole-based compound in human non-small cell lung cancer A549 cells. Biochem Pharmacol 66:115–124. https://doi.org/10.1016/S0006-2952(03)00254-5
Matysiak J, Opolski A (2006) Synthesis and antiproliferative activity of N-substituted 2-amino-5-(2,4-dihydroxyphenyl)-1,3,4-thiadiazoles. Bioorg Med Chem 14:4483–4489. https://doi.org/10.1016/j.bmc.2006.02.027
Wei M, Feng L, Li X, Zhou X, Shao Z (2009) Synthesis of new chiral 2,5-disubstituted 1,3,4-thiadiazoles possessing γ-butenolide moiety and preliminary evaluation of in vitro anticancer activity. Eur J Med Chem 44:3340–3344. https://doi.org/10.1016/j.ejmech.2009.03.023
Sun S, Yang Y, Li W, Zhang Y, Wang X, Tang J, Zhu H (2011) Synthesis, biological evaluation and molecular docking studies of 1,3,4-thiadiazole derivatives containing 1,4-benzodioxan as potential antitumor agents. Bioorg Med Chem Lett 21:6116–6121. https://doi.org/10.1016/j.bmcl.2011.08.039
Moshaf MH, Sorkhi M, Emami S, Nakhjiri M, Yahya-Meymandi A, Negahbani AS, Siavoshi F, Omrani M, Alipour E, Vosooghi M, Shafiee A, Foroumadi A (2011) 5-Nitroimidazole-based 1,3,4-thiadiazoles: heterocyclic analogs of metronidazole as anti-helicobacter pylori agents. Arch Pharm Chem Life Sci 344:178–183. https://doi.org/10.1002/ardp.201000013
Mirzaei J, Siavoshi F, Emami S, Safari F, Khoshayand MR, Shafiee A, Foroumadi A (2008) Synthesis and in vitro anti-helicobacter pylori activity of N-[5-(5-nitro-2-heteroaryl)-1,3,4-thiadiazol-2-yl]thiomorpholines and related compounds. Eur J Med Chem 43:1575–1580. https://doi.org/10.1016/j.ejmech.2007.11.019
Rajak H, Deshmukh R, Aggarwal N, Kashaw S, Kharya MD, Mishra P (2009) Synthesis of novel 2,5-disubstituted 1,3,4-thiadiazoles for their potential anticonvulsant activity: pharmacophoric model studies. Arch Pharm Chem Life Sci 342:453–461. https://doi.org/10.1002/ardp.200800213
Hamad NS, Al-Haidery NH, Al-Masoudi IA, Sabri M, Sabri L, Al-Masoudi NA (2010) Amino acid derivatives, part 4: synthesis and anti-HIV activity of new naphthalene derivatives. Arch Pharm Chem Life Sci 343:397–403. https://doi.org/10.1002/ardp.200900293
Ijichi K, Fujiwara M, Nagano H, Matsumoto Y, Hanasaki Y, Ide T, Katsuura K, Takayama H, Shirakawa S, Aimi N, Shigeta S, Konno K, Matsushima M, Yokota T, Baba M (1996) Anti-HIV-1 activity of thiadiazole derivatives: structure-activity relationship, reverse transcriptase inhibition, and lipophilicity. Antivir Res 31:87–94. https://doi.org/10.1016/0166-3542(96)00950-3
Sneader W (2005) Drug discovery: a history. Wiley, West Sussex. https://doi.org/10.1002/0470015535
Iyer G, Bellantone R, Taft D (1999) In vitro characterization of the erythrocyte distribution of methazolamide: a model of erythrocyte transport and binding kinetics. J Pharmacokinet Biopharm 27:45–66. https://doi.org/10.1023/A:1020630712388
Hall BS, Wilkinson SR (2012) Activation of benznidazole by trypanosomal type I nitroreductases results in glyoxal formation. Antimicrob Agents Chemother 56:115–123. https://doi.org/10.1128/AAC.05135-11
Messer WS (2002) The utility of muscarinic agonists in the treatment of Alzheimer’s disease. J Mol Neurosci MN 19:187–193. https://doi.org/10.1007/s12031-002-0031-5
Hansch C, Sammes PG, Taylor JB (1990) Comprehensive medicinal chemistry, vol 2. Pergamon Press, Oxford, Chap 7.1
Supuran Claudiu T (2017) Special issue: sulfonamides. Molecules 22:1642–1646. https://doi.org/10.3390/molecules22101642
Iqbal Z, Hameed S, Ali S, Tehseen Y, Shahid M, Iqbal J (2015) Synthesis, characterization, hypoglycemic and aldose reductase inhibition activity of arylsulfonylspiro[fluorene-9,5′-imidazolidine]-2′,4′-diones. Eur J Med Chem 98:127–138. https://doi.org/10.1016/j.ejmech.2015.05.011
Abbas MA, Hameed S, Farman M, Kressler J, Mahmood N (2015) Conjugates of degraded and oxidized hydroxyethyl starch and sulfonylureas: synthesis, characterization, and in vivo antidiabetic Activity. Bioconj Chem 26:120–127. https://doi.org/10.1021/bc500509a
Khan MH, Hameed S, Farman M, Al-Masoudi NA, Stoeckli-Evans HZ (2015) Synthesis, anti-HIV activity and molecular modeling study of 3-aryl-6-adamantylmethyl-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazole derivatives. Z Naturforsch 70b:609–616. https://doi.org/10.1515/znb-2015-0032
Willker W, Leibfritz D, Kerssebaum R, Bermel W (1993) Gradient selection in inverse heteronuclear correlation spectroscopy. Magn Reson Chem 31:287–292. https://doi.org/10.1002/mrc.1260310315
Pannecouque C, Daelemans D, De Clercq E (2008) Tetrazolium-based colorimetric assay for the detection of HIV replication inhibitors:revisited, 20 years later. Nat Protoc 3:427–434. https://doi.org/10.1038/nprot.2007.517
Hargrave KD, Proudfoot JR, Grozinger KG, Cullen E, Kapadia SR, Patel UR, Fuchs VU, Mauldin SC et al (1991) J Med Chem 34:2231–2241. https://doi.org/10.1021/jm00111a045
Mitsuya H, Weinhold KJ, Furman PA, St Clair MH, Lehrmann SN, Gallo RC, Bolognesi D, Barry DW, Broder S (1985) 3′-Azido-3′-deoxythymidine (BW A509U), an antiviral agent that inihibits the ineffectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associates virus in vitro. Proc Natl Acad Sci USA 82:7096–7100
Coates JA, Cammack N, Jenkinson HJ, Jowett AJ, Pearson BA, Penn CR, Rouse PL, Viner KC, Cameron JM (1992) The separated enantiomers of 2′-deoxy-3′-thiacytidine (BCH 189) both inhibit human immunodeficiency virus replication in vitro. Antimicrob Agents Chemother 36:733–739. https://doi.org/10.1128/AAC.36.4.733
Popovic M, Sarngadharan MG, Read E, Gallo RC (1984) Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science 224:497–500. https://doi.org/10.1126/science.6200935
Barré-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, Gruest J, Dauguet C, Axler-Blin C, Vezinet-Brun F, Rouzioux C, Rozenbaum W, Montagnier L (1983) Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 220:868–871. https://doi.org/10.1126/science.6189183
Miyoshi I, Taguchi H, Kobonishi I, Yoshimoto S, Ohtsuki Y, Shiraishi Y, Akagi T (1982) Type C virus-producing cell lines derived from adult T cell leukemia. Gann Monogr Cancer Res 28:219–228
Acknowledgements
We thank Prof. C. Pannecouque of Rega Institute for Medical Research, Katholieke Universiteit, Leuven, Belgium, for the anti-HIV screening.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Shafique, M., Hameed, S., Naseer, M.M. et al. Synthesis of new chiral 1,3,4-thiadiazole-based di- and tri-arylsulfonamide residues and evaluation of in vitro anti-HIV activity and cytotoxicity. Mol Divers 22, 957–968 (2018). https://doi.org/10.1007/s11030-018-9851-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-018-9851-2