Abstract
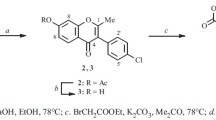
Isoflavones were synthesized by two steps in good yields, starting from commercially available 2-hydroxyacetophenones and benzene analogs. First, intermediate 3-(dimethylamino)-1-(2-hydroxyphenyl) prop-2-en-1-ones were obtained by the condensation of 2-hydroxyacetophenones and DMF-DMA in DMF with high yields. Second, isoflavones were synthesized by irradiation of 3-(dimethylamino)-1-(2-hydroxyphenyl)prop- 2-en-1-ones in the presence of iodine using benzene analogs as solvent under a mercury lamp.
Graphical Abstract






Similar content being viewed by others
References
Protti S, Manzini S, Fagnoni M, Albini A (2009) The contribution of photochemistry to green chemistry. In: Ballini R (ed) Eco-Friendly Synthesis of Fine Chemicals. RSC Green Chemistry Series. Royal Society of Chemistry, Cambridge, UK, pp 80–111
Harrison IT, Lythgoe B (1958) Calciferol and its relatives III, Partial synthesis of calciferol and of epicalciferol. J Chem Soc 837–843: doi:10.1039/jr9580000837
Jacobs HJC, Havinga E (1979) Photochemistry of vitamin D and its isomers and of simple trienes. Adv Photochem 11:305–373. doi:10.1002/9780470133415.ch4
Crossland NM, Roberts SM, Newton RF, Webb CF (1978) Synthesis of prostaglandin E\(_{2}\) and prostaglandin C\(_{2}\) from 5-endo,7-anti-disubstituted bicyclo[2.2.1]heptan-2-ones. J C S Chem Comm 660–661. doi:10.1039/c39780000660
Masters JJ, Jung DK, Danishefsky SJ et al (1995) A novel intramolecular Heck reaction: synthesis of a cholesterol-baccatin III hybrid. Angew Chem Int Edit 34:452–455. doi:10.1002/anie.199504521
Ji SJ, Takahashi E, Takahashi TT, Horiuchi CA (1999) Synthesis of \(\alpha, \beta \)-unsaturated ketones from \(\alpha \)-iodo ketones using photoirradiation. Tetrahedron Lett 40:9263–9266. doi: 10.1016/S0040-4039(99)02010-9
Barton DH, Basu NK, Day MJ, Hesse RH, Pechet MM, Starratt AN (1975) Improved syntheses of aldosterone. J Chem Soc Perkin Trans I:2243–2251
Blank VC, Poli C, Marder M, Roguin LP (2004) Antiproliferative activity of various flavonoids and related compounds: additive effect of interferon-\(\alpha \)2b. Med Chem Lett 14:133–136. doi: 10.1016/j.bmcl.2003.10.029
Li XC, Joshi AS, El-Sohly HN, Khan SI, Jacob MR, Zhang ZZ, Khan IA, Ferreira D, Walker LA, Broedel SE, Raulli RE, Cihlar RL (2002) Fatty acid synthase inhibitors from plants: isolation, structure elucidation, and SAR studies. J Nat Prod 65:1909–1914. doi:10.1021/np020289t
Damrongkiet A, Jisnuson S, Prasat K, Daraporn P, Morakot T, Yodhathai T (2002) Antiviral isoflavonoid sulfate and steroidal glycosides from the fruits of Solanum torvum. Phytochemistry 59:459–463
Laupattarakasem P, Houghton PJ, Robin J, Hoult S (2004) Anti-inflammatory isoflavonoids from the stems of Derris scandens. Planta Med 70:496–501. doi:10.1055/s-2004-827147
Kang KA, Zhang R, Piao MJ, Ko DO, Wang ZH, Kim BJ, Park JW, Kim HS, Kim DH, Hyun JW (2008) Protective effect of irisolidone, a metabolite of kakkalide, against hydrogen peroxide induced cell damage via antioxidant effect. Bioorg Med Chem 16:1133–1141. doi:10.1016/j.bmc.2007.10.085
Qin CX, Chen XQ, Hughes RA, Williams SJ, Woodman OL (2007) Understanding the cardioprotective effects of flavonols: discovery of relaxant flavonols without antioxidant activity. J Med Chem 51:1874–1884. doi:10.1021/jm070352h
Middleton E, Kandaswami C, Theoharides TC (2000) The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol Rev 52:673–751
Balasubramanian S, Nair MG (2000) An efficient “One Pot” synthesis of isoflavones. Synth Commun 30:469–484. doi:10.1080/00397910008087343
Zhang Q, Botting NP (2004) The synthesis of [2,3,4–13C3]glycitein. Tetrahedron 60:12211–12216. doi:10.1016/j.tet.2004.10.028
Prakash O, Pahuja S, Goyal S, Sawhney SN, Moriarty RM (1990) 1,2-Aryl shift in the hypervalent iodine oxidation of flavanones: A new useful synthesis of isoflavones. Synlett 6:337–338. doi:10.1055/s-1990-21084
Marais JPJ, Ferreira D, Slade D (2005) Stereoselective synthesis of monomeric flavonoids. Phytochemistry 66:2145–2176. doi:10.1016/j.phytochem.2005.03.006
Paguette LA, Stucki H (1966) A new general approach to the synthesis of oxygen-containing heterocycles by virtue of hydroxyl neighboring group participation. The condensation of enamines with salicylaldehydes. J Org Chem 31:1232–1235
Yokoe I, Sugita Y, Shirataki Y (1989) Facile synthesis of isoflavones by the cross-coupling reaction of 3-iodochromone with arylboronic acids. Chem Pharm Bull 37:529–530
Vasselin DA, Westwell AD, Matthews CS, Bradshaw TD, Stevens MFG (2006) Structural studies on bioactive compounds. Synthesis and biological properties of fluoro-, methoxyl-, and amino-substituted 3-phenyl-4H-1-benzopyran-4-ones and a comparison of their antitumor activities with the activities of related 2-phenylbenzothiazoles. J Med Chem 49:3973–3981. doi:10.1021/jm060359j
Rao MLN, Venkatesh V, Jadhav DN (2009) Pd-Catalyzed efficient cross-couplings of 3-iodochromones with triaryl-bismuths as substoichiometric multicoupling organometallic nucleophiles. Synlett 16:2597–2600. doi:10.1055/s-0029-1217959
Klier L, Bresser T, Nigst TA, Karaghiosoff K, Knochel P (2012) Lewis acid-triggered selective zincation of chromones, quinolones, and thiochromones: application to the preparation of natural flavones and isoavones. J Am Chem Soc 134:13584–13587. doi:10.1021/ja306178q
Zhang ZT, Li CC, Liu LZ, Wang QY, Xue D (2013) From faming zhuanli shenqing, 103265518. Abstr CA 159:426273
Gammill RB (1979) A new and efficient synthesis of 3-halogenated 4H–1-benzopyran-4-ones. Synthesis 11:901–903. doi:10.1055/s-1979-28869
Alexandersen P, Toussaint A, Christiansen C, Devogelaer JP, Roux C, Fechtenbaum J, Gennari C, Reginster JY (2001) Ipriflavone in the treatment of postmenopausal osteoporosis: A randomized controlled trial. JAMA 285:1482–1488. doi:10.1001/jama.285.11.1482
D’Auria M, De Luca E, Mauriello G, Racioppi R (1998) Photochemical synthesis of 4(5)-nitro-2-arylimidazoles. J Chem Soc Perkin Trans 1:271–273
Acknowledgments
This research was supported by the National Natural Science Foundation of China (No. 21372150), the Fundamental Research Funds for the Central Universities (No. GK261001095), and the Science and Innovation Funds of Graduate Programs of Shaanxi Normal University (No. 2009CXS013).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, L., Wang, Q., Zhang, Z. et al. An efficient strategy to syntheses of isoflavones. Mol Divers 18, 777–785 (2014). https://doi.org/10.1007/s11030-014-9537-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-014-9537-3