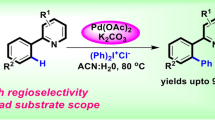
Abstract
An efficient palladium-catalyzed strategy through C–H bond activation for the synthesis of 2(2\(^{\prime }\)-biphenyl)-benzimidazoles is reported herein. The regioselective C–C bond formation proceeds in a sealed tube via oxidative C–H activation of ortho-directed 2-aryl-benzimidazole to couple with various iodobenzene analogs in high yields. This arylation exhibited high regioselectivity which is able to increase molecular diversity in difficult functionalized positions of parent molecules. This strategy provides a convenient and simple synthesis of biphenyl heterocyclic compounds with high regioselectivity.
Graphical Abstract






Similar content being viewed by others
References
Yu JQ, Shi ZJ (2010) C-H activation. Top Curr Chem Springer Berlin Heidelberg 292: xi-xiii. doi:10.1007/978-3-642-12356-6
Gunay A, Theopold KH (2010) C–H bond activations by metal oxo compounds. Chem Rev 110:1060–1081. doi:10.1021/cr900269x
Ackermann L (2011) Carboxylate-assisted transition-metal-catalyzed C-H bond functionalizations: mechanism and scope. Chem Rev 111:1315–1345. doi:10.1021/cr100412j
Alberico D, Scott ME, Lautens M (2007) Aryl–aryl bond formation by transition-metal-catalyzed direct arylation. Chem Rev 107: 174–238. doi:10.1021/cr0509760
Chen X, Engle KM, Wang DH, Yu JQ (2009) Palladium(II)-catalyzed C–H activation/C–C cross-coupling reactions: versatility and practicality. Angew Chem Int Ed 48:5094–5115. doi:10.1002/anie.200806273
Cardenas DJ, Martin-Matute B, Echavarren AM (2006) Aryl transfer between Pd(II) centers or Pd(IV) intermediates in Pd-catalyzed domino reactions. J Am Chem Soc 128:5033–5040. doi:10.1021/ja056661j
Corbet JP, Mignani G (2006) Selected patented cross-coupling reaction technologies. Chem Rev 106:2651–2710. doi:10.1021/cr0505268
Jana R, Pathak TP, Sigman MS (2011) Advances in transition metal (Pd, Ni, Fe)-catalyzed cross-coupling reactions using alkyl-organometallics as reaction partners. Chem Rev 111:1417–1492. doi:10.1021/cr100327p
Ritleng V, Sirlin C, Pfeffer M (2002) Ru-, Rh-, and Pd-catalyzed C–C bond formation involving C–H activation and addition on unsaturated substrates?: reactions and mechanistic aspects. Chem Rev 102:1731–1770. doi:10.1021/cr0104330
Sun CL, Li BJ, Shi ZJ (2011) Direct C–H transformation via iron catalysis. Chem Rev 111:1293–1314. doi:10.1021/cr100198w
Zhang J, Yang Q, Zhu Z, Yuan ML, Fu HY, Zheng XL, Chen H, Li RX (2012) Acetamide as cocatalyst for the nitrogen-directed coupling of arenes with aryl chlorides through ruthenium-catalyzed C–H activation. Eur J Org Chem 6702–6706. doi:10.1002/ejoc.201201127
Daugulis O, Zaitsev VG (2005) Anilide ortho-arylation by using C–H activation methodology. Angew Chem Int Ed 44:4046–4048. doi:10.1002/anie.200500589
Rousseaux S, Gorelsky S, Chung BKW, Fagnou K (2010) Investigation of the mechanism of C(sp\(^{3})\)–H bond cleavage in Pd(0)-catalyzed intramolecular alkane arylation adjacent to amides and sulfonamides. J Am Chem Soc 132:10692–10705. doi: 10.1021/ja103081n
Wang GW, Yuan TT, Wu XL (2008) Direct ortho-acetoxylation of anilides via palladium-catalyzed sp\(^{2}\) C–H bond oxidative activation. J Org Chem 71:4717–4720. doi: 10.1021/jo8003088
Dupont J, Consorti CS, Spencer J (2005) The potential of palladacycles?: more than just precatalysts. Chem Rev 105:2527–2572. doi:10.1021/cr030681r
Yang S, Li B, Wan X, Shi Z (2007) Ortho arylation of acetanilides via Pd(II)-catalyzed C–H functionalization. J Am Chem Soc 129:6066–6067. doi:10.1021/ja070767s
Chen X, Goodhue CE, Yu JQ (2008) Palladium-catalyzed alkylation of sp\(^{2}\) and sp\(^{3}\) C–H bonds with methylboroxine and alkylboronic acids?: two distinct C–H activation pathways. J Am Chem Soc 128:12634–12635. doi: 10.1021/ja0646747
Lyons TW, Sanford MS (2010) Palladium-catalyzed ligand-directed C–H functionalization reactions. Chem Rev 110: 1147–1169. doi:10.1021/cr900184e
Ozkay Y, Tunali Y, Karaca H, Isikdag I (2010) Antimicrobial activity and a SAR study of some novel benzimidazole derivatives bearing hydrazones moiety. Eur J Med Chem 45:3293–3298. doi:10.1016/j.ejmech.2010.04.012
Rewcastle GW, Gamage SA, Flanagan JU, Frederick R, Denny WA, Baguley BC, Kestell P, Singh R, Kendall JD, Marshall ES, Lill CL, Lee WJ, Kolekar S, Buchanan CM, Jamieson SMF, Shepherd PR (2011) Synthesis and biological evaluation of novel analogues of the pan class I phosphatidylinositol 3-kinase (PI3K) inhibitor 2-(difluoromethyl)-1-[4,6-di (4-morpholinyl)-1,3,5-triazin-2-yl]-1\(H\)-benzimidazole (ZSTK474). J Med Chem 54:7105–7126. doi:10.1021/jm200688y
Anthony NJ, Gomez RP, Stokker GE, Wai JS, Williams TM, Halczenko W, Hutchinson JH, Young SD, Solinsky KM (2000) US Patent 00608070A
Charton J, Girault-Mizzi S, Debreu-Fontaine MA, Foufelle F, Hainault I, Bizot-Espiard JG, Caignard DH, Sergheraert C (2006) Synthesis and biological evaluation of benzimidazole derivatives as potent AMP-activated protein kinase activators. Bioorg Med Chem 14:4490–4518. doi:10.1016/j.bmc.2006.02.028
Tyagarajan S, Chakravarty PK, Zhou B, Fisher MH, Wyvratt MJ, Lyons K, Klatt T, Li X, Kumar S, Williams B, Felix J, Priest BT, Brochu RM, Warren V, Smith M, Garcia M, Kaczorowski GJ, Martin WJ, Abbadie C, McGowan E, Jochnowitz N, Parsons WH (2010) Substituted biaryl oxazoles, imidazoles, and thiazoles as sodium channel blockers. Bioorg Med Chem Lett 20:5536–5540. doi:10.1016/j.bmcl.2010.07.064
Benson SC, Pershadsingh H, Ho C, Chittiboyina A, Desai P, Pravenec M, Qi N, Wang J, Avery MA, Kurtz TW (2004) Identification of Telmisartan as a unique angiotensin II receptor antagonist with selective PPAR-modulating activity. Hypertension 43: 993–1002. doi:10.1161/01.HYP.0000123072.34629.57
Kubo K, Kohara Y, Imamiya E, Sugiura Y, Inada Y, Furukawa Y, Nishikawa K, Naka T (1993) Nonpeptide angiotensin II receptor antagonists. Synthesis and biological activity of benzimidazolecarboxylic acids. J Med Chem 36:2182–2195. doi:10.1021/jm00067a016
Bali A, Bansal Y, Sugumaran M, Saggu JS, Balakumar P, Kaur G, Bansal G, Sharma A, Singh M (2005) Design, synthesis, and evaluation of novelly substituted benzimidazole compounds as angiotensin II receptor antagonists. Bioorg Med Chem Lett 15:3962–3965. doi:10.1016/j.bmcl.2005.05.054
Kim Y, Kumar MR, Park N, Heo Y, Lee S (2011) Copper-catalyzed, one-pot, three-component synthesis of benzimidazoles by condensation and C–N bond formation. J Org Chem 76:9577–9583. doi:10.1021/jo2019416
Rosenberg AJ, Zhao J, Clark DA (2012) Synthesis of imidazo[4, 5-b]pyridines and imidazo[4,5-b]pyrazines by palladium catalyzed amidation of 2-chloro-3-aminoheterocycles. Org Lett 14: 1764–1767. doi:10.1021/ol300359s
Bellina F, Cauteruccio S, Rossi R (2006) Palladium- and copper-mediated direct C-2 arylation of azoles-including free (NH)-imidazole, -benzimidazole and -indole - under base-free and ligandless conditions. Eur J Org Chem 1379–1382. doi:10.1002/ejoc.200500957
Charton J, Girault-Mizzi S, Sergheraert C (2005) Conversion of sterically hindered diacylated 1,2-phenylenediamines into 2-substituted benzimidazoles. Chem Pharm Bull 35: 492–497
Stibrany RT, Lobanov MV, Schugar HJ, Potenza JA (2004) A geometrically constraining bis(benzimidazole) ligand and its nearly tetrahedral complexes with Fe(II) and Mn(II). Inorg Chem 43:1472–1480. doi:10.1021/ic030180o
Wang R, Lu XX, Yu XQ, Shi L, Sun Y (2007) Acid-catalyzed solvent-free synthesis of 2-arylbenzimidazoles under microwave irradiation. J Mol Catal A 266:198–201. doi:10.1016/j.molcata.2006.04.071
Blettner CG, Konig WA, Rühter G, Stenzel W, Schotten T (1999) Parallel synthesis of polyethylene glycol supported biaryl benzimidazoles and imidazopyridines. Synlett 3:307–310
Miyamura C, Tsurugi H, Satoh T, Miura M (2008) Rhodium-catalyzed regioselective arylation of phenylazoles and related compounds with arylboron reagents via C–H bond cleavage. J Organomet Chem 693:2438–2442. doi:10.1016/j.jorganchem.2008.04.029
Peng J, Shang G, Chen C, Miao Z, Li B (2013) Nucleophilic addition of benzimidazoles to alkynyl bromides/palladium-catalyzed intramolecular C–H vinylation: synthesis of benzo[4,5]imidazo[2,1-a]isoquinolines. J Org Chem 78:1242–1248. doi:10.1021/jo302471z
Yellol GS, Chung TW, Sun CM (2010) Novel cyclization of bis-boc-guanidines: expeditive traceless synthesis of 1,3,5-oxadiazinones under microwave conditions. Chem Commun 46:9170–9172. doi:10.1039/c0cc03519j
Chen CH, Yellol GS, Lin PT, Sun CM (2011) Base-catalyzed Povarov reaction: an unusual [1,3] sigmatropic rearrangement to dihydropyrimidobenzimidazoles. Org Lett 13:5120–5123. doi:10.1021/ol201985p
Bancroft DP, Cotton A, Verbruggen M (1989) Trans-(dimethyl sulfoxide-O) (dimethyl sulfoxide-S) bis(trifluoroacetato)palladium(II); alternative ligation modes of an ambidentate ligand. Acta Crystallogr C 45:1289–1292. doi:10.1107/S0108270189001459
Lirag RC, Ley HTM, Miljanic OS (2013) L-shaped benzimidazole fluorophores: synthesis, characterization and optical response to bases, acids and anions. Chem Commun 49:4304–4306. doi:10.1039/c2cc37120k
Acknowledgments
The authors wish to thank the National Science Council of Taiwan for financial support and the authorities of the National Chiao Tung University for providing the laboratory facilities. This study was particularly supported by the “Centre for Bioinformatics Research of Aiming for the Top University Plan” of the National Chiao Tung University and Ministry of Education, Taiwan.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, LH., Wu, TY., Paike, V. et al. Palladium-catalyzed regioselective synthesis of 2(2\(^{\prime }\)-biphenyl)benzimidazoles through C–H activation. Mol Divers 17, 641–649 (2013). https://doi.org/10.1007/s11030-013-9460-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-013-9460-z