Abstract
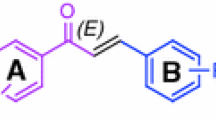
A new approach for the preparation of new 9-(alkyl or aryl)acenaphtho[1,2-b]furan-8-(alky or aryl) amine derivatives has been reported by the catalyst-free one-pot cyclocondensation of (acenaphthylen-1-yloxy)trimethylsilane, alkyl and aryl aldehydes, and aryl and alky isocyanides in refluxing DMF.
Similar content being viewed by others
References
Dean FM (1982) Recent advances in furan chemistry. Part I. Adv Heterocycl Chem 30: 167–238. doi:10.1016/S0065-2725(08)60400-6
Lipshutz BH (1986) Oxazoles in carboxylate protection and activation. Chem Rev 86: 795–822. doi:10.1021/cr00075a008
Teimouri MB, Mansouri F (2008) Simple synthesis of 7H-phenaleno[1,2-b]furan-7-one derivatives by one-pot, three-component reactions. J Comb Chem 10: 507–510. doi:10.1021/cc8000103
Sternbach DD, Rossana DM (1982) Intramolecular diels-alder reactions of the furan diene: substituent and solvent effects. Tetrahedron Lett 23: 303–306. doi:10.1016/S0040-4039(00)86815-X
Baghernejad B, Heravi MM, Oskooie HA, Poormohammad N, Khorshidi M, Beheshtiha YS (2011) A novel and facile synthesis of N-cyclohexyl-benzofurans by one-pot three-component reaction. Mol Divers 15: 245–248. doi:10.1007/s11030-010-9228-7
Trost BM (1991) The atom economy—a search for synthetic efficiency. Science 254: 1471–1477. doi:10.1126/science.1962206
Bienaymé H., Hulme C., Oddon G., Schmitt P (2000) Maximizing synthetic efficiency: multi-component transformations lead the way. Chem Eur J 6: 3321–3329. doi:10.1002/1521-3765(20000915)
Orru RVA, De Greef M (2003) Recent advances in solution-phase multicomponent methodology for the synthesis of heterocyclic compounds. Synthesis 1471–1499. doi:10.1055/s-2003-40507
Von Wangelin AJ, Neumann H, Gördes D, Klaus S, Strübing D, Beller M (2003) Multicomponent coupling reactions for organic synthesis: chemoselective reactions with amide-aldehyde mixtures. Chem Eur J 9: 4286–4294. doi:10.1002/chem.200305048
Kobayashi S (1999) New methodologies for the synthesis of compound libraries. Chem Soc Rev 28: 1–15. doi:10.1039/A707429H
Tejedor D, González-Cruz D, Santos-Expósito A, Marrero- Tellado JJ, de Armas P, García-Tellado F (2005) Multicomponent domino processes based on the organocatalytic generation of conjugated acetylides: efficient synthetic manifolds for diversity-ori- ented molecular construction. Chem-Eur J 11: 3502–3510. doi:10.1002/chem.200401267
Zhu J (2003) Recent developments in the isonitrile-based multicomponent synthesis of heterocycles. Eur J Org Chem 1133–1144. doi:10.1002/ejoc.200390167
Tietze LF, Modi A (2000) Multicomponent domino reactions for the synthesis of biologically active natural products and drugs. Med Res Rev 20: 304–322. doi:10.1002/1098-1128(200007)
Ugi I, Dömling A, Hörl W (1994) Multicomponent reactions in organic chemistry. Endeavour 18: 115–122. doi:10.1016/S0160-9327(05)80086-9
Ugi I, Domling A, Werner B (2000) Since 1995 the new chemistry of multicomponent reactions and their libraries, including their heterocyclic chemistry. J Heterocycl Chem 37: 647–658. doi:10.1111/1475-4983.00314
Passerini M (1922) Natural product synthesis using multicomponent reaction strategies. Gazz Chim Ital 52: 432–435
Ugi I, Steinbrückner C (1960) Über ein neues Kondensations-Prinzip. Angew Chem 72: 267–268. doi:10.1002/ange.19600720709
Ugi I., Meyr R., Fetzer U., Steinbrückner C (1959) Versuche mit Isonitrilen Angew Chem 71: 386–388. doi:10.1002/ange.19590711110
Ugi I, Lohberger S, Karl R (1991) The Passerini and Ugi reactions. Compr Org Synth 2: 1083–1109. doi:10.1016/B978-0-08-052349-1.00057-3
Murakami M (2003) New catalyzed three-component cycloadditions for the synthesis of eight-membered carbocycles. Angew Chem Int Ed 42: 718–720. doi:10.1002/anie.200390200
Marcaccini S, Torroba T (1993) The use of isocyanides in heterocyclic synthesis. Org Prep Proced Int 25: 141–208. doi:10.1080/00304949309457947
Hulme C, Gore V (2003) Multi-component reactions: Emerging chemistry in drug discovery from Xylocain to Crixivan. Curr Med Chem 10: 51–80. doi:10.2174/0929867033368600
Dömling A, Ugi I (2000) Multicomponent reactions with isocyanides. Angew Chem Int Ed 39: 3169–3210. doi:10.1002/1521-3773(20000915)
Dömling A (2002) Recent advances in isocyanide-based multicomponent chemistry. Curr Opin Chem Biol 6: 306–313. doi:10.1016/S1367-5931(02)00328-9
Cristau P, Vors JP, Zhu J (2006) Rapid synthesis of cyclopeptide alkaloid-like para-cyclophanes by combined use of Ugi-4CR and intramolecular SNAr reaction. QSAR Comb Sci 25: 519–526. doi:10.1002/qsar.200540214
Balme G, Bossharth E, Monteiro N (2003) Pd-assisted multicomponent synthesis of heterocycles. Eur J Org Chem 4101–4111. doi:10.1002/ejoc.200300378
Nair V, Menon RS, Vinod AU, Viji S (2002) A facile three-component reaction involving [4+1] cycloaddition leading to furan annulated heterocycles. Tetrahedron Lett 43: 2293–2295. doi:10.1016/S0040-4039(02)00226-5
Shaabani A, Teimouri MB, Bijanzadeh HR (2002) One-pot three component condensation reaction in water: An efficient and improved procedure for the synthesis of furo[2,3-d]pyrimidine-2,4(1H,3H)-diones. Tetrahedron Lett 43: 9151–9154. doi:10.1016/S0040-4039(02)02260-8
Shaabani A, Teimouri MB, Bijanzadeh HR (2004) A novel three-component tetrahydrobenzofuran synthesis. Monatsh Chem 135: 441–446. doi:10.1002/chin.200435097
Shaabani A, Teimouri MB, Bijanzadeh HR (2004) One-pot three-component condensation reactions in water. An efficient and improved procedure for the synthesis of furan annulated heterocycles. Monatsh Chem 135: 589–593. doi:10.1007/s00706-003-0126-x
Shaabani A, Teimouri MB, Samadi S, Soleimani K (2005) Microwave-assisted three-component condensation on montmorillonite K10: Solvent-free synthesis of furopyrimidines, furocoumarins, and furopyranones. Synth Commun 35: 535–541. doi:10.1002/chin.200531116
Teimouri MB, Bazhrang R (2006) Shaken not stirred: a facile synthesis of 1,4-bis(furo[2,3-d]-pyrimidine-2,4(1H,3H)-dione-5-yl) benzenes by one-pot reaction of isocyanides, N,N′-dimethylbarbituric acid, and terephthaldialdehyde. Bioorg Med Chem Lett 16: 3697–3701. doi:10.1016/j.bmcl.2006.04.065
Teimouri MB, Khavasi HR (2007) One-pot three-component regioselective synthesis of linear naphtho[2,3-b]-furan-4,9-diones. Tetrahedron 63: 10269–10275. doi:10.1016/j.tet.2007.07.082
Song JJ, Tan Z, Reeves JT, Fandrick DR, Yee NK, Senanayake CH (2008) N-heterocyclic carbene-catalyzed silyl enol ether for- mation. Org Lett 10: 877–880. doi:10.1021/ol703025b
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
The Below is the Electronic Supplementary Material.
Rights and permissions
About this article
Cite this article
Sandaroos, R., Damavandi, S., Salimi, M. et al. New approach for the synthesis of novel acenaphtho[1,2-b]furan-8-amines. Mol Divers 16, 269–277 (2012). https://doi.org/10.1007/s11030-012-9356-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-012-9356-3