Abstract
Recent studies indicated that apart from lysosomal storage of glycosaminoglycans (GAGs), secondary and tertiary changes in cellular processes may significantly contribute to development of disorders and symptoms occurring in mucopolysaccharidoses (MPS), a group of lysosomal storage diseases in which neurodegeneration is specific for most types and subtypes. In this report, using transcriptomic data, we demonstrate that regulation of hundreds of genes coding for proteins involved in regulations of various cellular processes is changed in cells derived from patients suffering from all types and subtypes of MPS. Among such genes there are 10 which expression is significantly changed in 9 or more (out of 11) MPS types/subtypes; they include IER3IP1, SAR1A, TMEM38B, PLCB4, SIN3B, ABHD5, SH3BP5, CAPG, PCOLCE2, and MN1. Moreover, there are several genes whose expression is changed over log2 > 4 times in some MPS types relative to control cells. The above analysis indicates that significant changes in expression of genes coding for various regulators of cellular processes may considerably contribute to development of cellular dysfunctions, and further appearance of specific symptoms of MPS, including neurodegeneration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mucopolysaccharidoses (MPS) are a group of inherited metabolic diseases (Zhou et al. 2020). They belong to lysosomal storage disorders (LSD) (Sun 2018) since the primary cause of them is a lack or significant decrease in activity of one of enzymes involved in degradation of glycosaminoglycans (GAGs). Due to the presence of specific mutations and enzymatic dysfunctions, undegraded GAGs accumulate in lysosomes and cause defects in cellular functions. There are 11 types and subtypes of MPS, depending on the kind of stored GAG(s) and enzymatic defect (Kubaski et al. 2020). In 7 out of 11 types/subtypes, central nervous system (CNS) is involved, due to severe neurodegenerative processes occurring in the course of the diseases (Kobayashi 2019). In fact, all MPS types are severe diseases with progressing symptoms appearing in virtually all tissues and organs (especially in neuronopathic forms). The average life span is estimated to about two decades (Kobayashi 2019; Zhou et al. 2020).
Initially, GAG storage was considered as the only cause of the disease (Dorfman and Matalon 1976; Kelly 1976). However, subsequent studies indicated that secondary and tertiary changes in cells might significantly contribute to development of the disease symptoms (Gaffke et al. 2019; Fecarotta et al. 2020). Very recent transcriptomic analyses indicated that there are hundreds of genes whose transcription is down- or up-regulated in MPS cells relative to control cells (Gaffke et al. 2020). These studies were based on the use of lines of fibroblasts derived from patients suffering from all types and subtypes of MPS. Despite obvious limitations of such experiments, like the use of cell types which cannot represent most of tissues in patients’ bodies, and representation of each MPS type/subtype by only one cell lines, the advantages of these studies were possibility to compare transcriptomic changes in all MPS types/subtypes in one experiment and under the same conditions, and possibility to identify genes whose expression is significantly changed in most types/subtypes. In such a way, it was possible to preliminarily identify the genes whose changed expression in MPS cells might be responsible for or contribute to disorders in various cellular processes, including apoptosis (Brokowska et al. 2020) and cell activation (Rintz et al. 2020), or even to disturbance in behavioral disorders (Pierzynowska et al. 2020). This has encouraged us to ask what genes coding for proteins involved in regulation of cellular processes reveal changed expression in MPS cells relative to controls. Therefore, results of relevant transcriptomic analyses are presented in this report.
Materials and methods
Analyses performed in this report were based on the use of the RNA-seq data, deposited in the NCBI Sequence Read Archive (SRA), under accession number PRJNA562649 (Gaffke et al. 2020). The data were obtained on the basis of four biological repeats (understood as four independent experiments with one cell line at different passages, between 4th and 15th) of the experiment with fibroblasts derived from a healthy person and patients suffering from all known types and subtypes of MPS (Table 1). As described previously (Gaffke et al. 2020), Illumina TruSeq Stranded mRNA Library Prep Kit was used to prepare the mRNA libraries. Then, the cDNA libraries were sequenced employing a HiSeq4000 (Illumina, San Diego, CA, USA). Following parameters were used: PE150 (150 bp paired-end) and minimum 40 × 106 of raw reads. This gave a minimum of 12 Gb of raw data per each sample. FastQC version v0.11.7 was used for quality assessment. Raw readings were mapped to the GRCh38 human reference genome from the Ensembl database. Hisat2 v. 2.1.0 program was used for this procedure. Cuffquant and Cuffmerge programs in version 2.2.1 and the GTF Homo_sapiens.GRCh38.94.gtf file from the Ensembl database were used to calculate the expression levels. The Cuffmerge program was started with the library-norm-method classic-fpkm parameter normalizing the expression values by means of the FPKM algorithm. Statistical significance was analyzed using one-way analysis of variance (ANOVA) on log2(1 + x) values which have normal continuous distribution. The false discovery rate (FDR) was estimated using the Benjamini–Hochberg method. For comparisons between two groups, post hoc Student’s t test with Bonferroni correction was employed. R software v3.4.3 was employed to conduct all statistical analyses. Transcript annotation and classification was performed using the BioMart interface for the Ensembl gene database.
Results
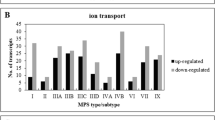
Using transcriptomic data obtained from fibroblasts derived from patients suffering from all types and subtypes of MPS, as well as control fibroblasts, we have analyzed transcripts with significantly changed levels in MPS relative to the control, derived from genes listed in the Ensembl database in the term ‘regulation of cellular process’ (GO:0050794), according to the Gene Ontology Consortium. We found that there were hundred or more up- and down-expressed transcripts coding for proteins involved in the regulation of cellular processes in each MPS type/subtype (Fig. 1). The changes appeared more abundant in MPS types I, III (all subtypes), IVB, VII and IX than in types II, IVA and VI. Numbers of up- and down-regulated transcripts were roughly equal in every types/subtype. These results suggest that regulation of cellular processes can be significantly changed in all types/subtypes of MPS, indeed.
In the next step, we have analyzed direct children terms of GO:0050794 (regulation of cellular process). Again, as depicted in Fig. 2, significant numbers of transcripts with considerably changed levels have been noted in all MPS types/subtypes when considering following GO terms: regulation of cellular metabolic process (GO:0031323), signal transduction (GO:0007165), positive regulation of cellular process (GO:0048522), negative regulation of cellular process (GO:0048523), regulation of cellular component organization (GO:0051128), regulation of cell communication (GO:0010646), regulation of cell death (GO:0010941), regulation of cell population proliferation (GO:0042127), regulation of cell cycle (GO:0051726), regulation of cellular component biogenesis (GO:0044087).
When assessing genes whose expression is significantly changed in most MPS types/subtypes, we have found that 10 genes are up- or down-regulated in at least 9 MPS types/subtypes (Table 2). These genes include IER3IP1, SAR1A, TMEM38B, PLCB4, SIN3B, ABHD5, SH3BP5, CAPG, PCOLCE2, and MN1. The first six genes from this group were down-regulated in all MPS types/subtypes while the last four genes were up-regulated in all MPS types/subtypes, indicating that there is a common pattern of expression dysregulation of these genes. Heat map representing these changes is shown in Fig. 3.
We have also analyzed genes whose expressions were especially highly changed in MPS cells relative to control fibroblasts. Thus, we assessed transcripts with log2 of fold change (FC) exceeding 2.5, 3.0, 3.5, 4.0. Numbers of such transcripts were significant in each of these groups (Fig. 4). The list of genes in which log2FC exceeds 4.0 in any MPS type/subtype includes: WISP2, RARRES2, APOE, TNFRSF11B, MMP3, CXCL8, PTGS1, WISP2, CAV1, SNX3, MMP12, CD9, COMP, TFPI2, IGFBP5, CAPG, OXTR, KRT19, CRLF1, CRIP1, and NME2 (Table 3).
Discussion
Recent studies clearly indicated that secondary and tertiary changes (after the primary GAG storage) in cellular processes contribute significantly to the development of disorders and symptoms appearing in the course of MPS (Gaffke et al. 2019; Fecarotta et al. 2020). Significance of this aspect of MPS pathomechanism has been highlighted recently by discoveries of multiple changes in expression of genes coding for proteins involved in various cellular mechanisms in MPS (Brokowska et al. 2020; Gaffke et al. 2020; Pierzynowska et al. 2020; Rintz et al. 2020). Therefore, in this work, we have analyzed transcriptomic data, focusing on expression of genes coding for regulatory proteins responsible for the control of cellular processes. We have used transcriptomic data based on analysis of biological samples derived from patients suffering from all types and subtypes of MPS. This allowed us to obtain a global picture of changes in levels of transcripts in MPS which is an advantage of such an experimental system. The obvious limitations were the use of one cell line from each MPS type/subtype (due to technical reasons), which cannot reflect a variability between patients suffering from the same MPS type, and employment of fibroblasts which represent only one type of cells. Nevertheless, confirmation of transcriptomic results, obtained for selected genes by the RT-qPCR method (reported previously by Gaffke et al. 2020; Pierzynowska et al. 2020; Rintz et al. 2020), as well as the fact that expression of particular genes was generally either up- or down-regulated in all/vast majority of MPS types/subtypes, strongly suggest that these analyses are reliable.
Here, we demonstrated that expression of hundreds of genes coding for regulators of cellular processes is changed in each MPS type/subtype. When assessing genes whose expression is significantly dysregulated, relative to control cells, in at least 9 MPS types/subtypes, following ten were found: IER3IP1, SAR1A, TMEM38B, PLCB4, SIN3B, ABHD5, SH3BP5, CAPG, PCOLCE2, and MN1.
IER3IP1 is the immediate early response 3 interacting protein 1. This protein is localized to the endoplasmic reticulum (ER), and it was suggested to play a role in the ER stress response. Possibly, this function can influence cell differentiation and apoptosis. Mutations in the IER3IP1 gene were connected to severe dysfunctions in CNS, and the symptoms include epilepsy (Valenzuela et al. 2017). Expression of IER3IP1 is down-regulated in all MPS types/subtypes, thus, one might suggest that lower levels of the gene product may contribute to neurological symptoms in MPS patients.
The SAR1A gene encodes secretion associated Ras-related GTPase 1A. This protein is involved in the transport from ER to the Golgi apparatus (Donaldson and Jackson 2011). Since SAR1A expression is impaired in all MPS types/subtypes, it is likely that SAR1A deficiency may partially cause the problems with vesicular transport, reported for MPS (for a review, see Gaffke et al. 2019).
The maintenance of intracellular calcium release is partially dependent on the function of the transmembrane protein 38B, a monovalent cation channel, encoded by the TMEM38B gene. Mutations in TMEM38B were connected to osteogenesis imperfecta (Valadares et al. 2014), and skeletal problems are common in MPS. Thus, decreased levels of TMEM38B transcripts in MPS cells may be partially responsible for such symptoms.
PLCB4 encodes phospholipase C β4. Formation of inositol 1,4,5-trisphosphate and diacylglycerol from phosphatidylinositol 4,5-bisphosphate is catalyzed by this enzyme. Therefore, decreased expression of PLCB4, observed in MPS, may impair intracellular transduction of many extracellular signals. In fact, dysregulation of PLCB4 signaling has been suggested to be linked to various brain disorders (Yang et al. 2016) which may indicate contribution of phospholipase C β 4 deficiency to neurodegeneration in MPS.
Transcription regulator family member B, encoded by the SIN3B gene, is involved in the regulation of cell cycle progression (Kadamb et al. 2013). Thus, significantly changed expression of this gene in cells of most MPS types/subtypes indicates that cell proliferation disturbance in MPS cell may partially result from improper levels of this transcription repressor.
The ABHD5 gene codes for one of abhydrolases. Mutations in ABHD5 are associated with a triglyceride storage disease (Missaglia et al. 2019). Therefore, one might presume that decreased expression of this gene in MPS may contribute to secondary storage, often observed in this disease.
The SH3 domain binding protein 5, encoded by SH3BP5, is a negative regulator of the phosphorylation activity of BTK, one of Tec family kinases (Ortutay et al. 2008). The SH3BP5 gene product may be involved in the control of apoptosis, thus, one can propose that it contributes to dysregulation of this process, reported in MPS (Brokowska et al. 2020).
The CAPG gene codes for capping actin protein, gelsolin like (Nag et al. 2013), contributing to the control of actin-based motility. Dysregulation of expression of this gelsolin-like protein may result in functional problems of actin cytoskeleton in MPS.
PCOLCE2 encodes procollagen C-endopeptidase enhancer 2 (Sorci-Thomas et al. 2015). Its enhanced expression in MPS cells may indicate disturbed regulation of collagen metabolism.
The MN1 gene encodes a transcription factor which may be involved in development of meningioma (Handschuh 2019). Therefore, enhanced expression of MN1 in MPS cells might potentially cause various defects in CNS.
The above analysis indicates that significant changes in expression of genes coding for various regulators of cellular processes may considerably contribute to the development of cellular dysfunctions, and further appearance of specific symptoms of MPS, including neurodegeneration. This conclusion may be supported by the analysis of genes whose expression is particularly severely dysregulated in some MPS types/subtypes. The list of such genes includes APOE (coding for apolipoprotein E), OXTR (coding for oxytocin receptor) and other genes coding for proteins involved in the control of crucial cellular processes. Further studies on molecular mechanisms of cellular changes related to disturbed expression of various genes should indicate specific processes which are dysregulated in MSP cells, leading to better understanding of pathomechanisms in this group of diseases.
In summary, transcriptomic analyses presented in this work not only indicated genes coding for proteins involved in regulation of cellular processes whose expression is significantly changed in fibroblasts of different MPS types (Fig. 1), including those affected in most types (Table 2, Fig. 3), but also suggested what processes may be especially dysregulated. These include metabolic processes, signal transduction, organization of cellular components, cell communication, cell death, cell population proliferation, cell cycle, and biogenesis of cellular components (Fig. 2). Interestingly, the genes with most severely changed expression were either up- or down-regulated in all or vast majority of MPS types (Table 3, Fig. 4), suggesting that there are groups of processes similarly changed in all these types, despite evident specificity of each type. Although experiments presented in this report were performed on fibroblasts, specific changes in expressions of regulatory proteins might, to some extent, be extrapolated to other cell types, including neurons as discussed previously (Pierzynowska et al. 2020), suggesting that they could contribute to neurodegenerative processes occurring in MPS.
Data availability
RNA-seq data, deposited in the NCBI Sequence Read Archive (SRA), are available under accession number PRJNA562649.
References
Brokowska J, Pierzynowska K, Gaffke L, Rintz E, Węgrzyn G (2020) Expression of genes involved in apoptosis is dysregulated in mucopolysaccharidoses as revealed by pilot transcriptomic analyses. Cell Biol Int in press. https://doi.org/10.1002/cbin.11332
Donaldson JG, Jackson CL (2011) ARF family G proteins and their regulators: roles in membrane transport, development and disease. Nat Rev Mol Cell Biol 12:362–375. https://doi.org/10.1038/nrm3117
Dorfman A, Matalon R (1976) The mucopolysaccharidoses (a review). Proc Natl Acad Sci U S A 73:630–637. https://doi.org/10.1073/pnas.73.2.630
Fecarotta S, Tarallo A, Damiano C, Minopoli N, Parenti G (2020) Pathogenesis of mucopolysaccharidoses, an update. Int J Mol Sci 21:2515. https://doi.org/10.3390/ijms21072515
Gaffke L, Pierzynowska K, Podlacha M, Brokowska J, Węgrzyn G (2019) Changes in cellular processes occurring in mucopolysaccharidoses as underestimated pathomechanisms of these diseases. Cell Biol Int in press. https://doi.org/10.1002/cbin.11275
Gaffke L, Pierzynowska K, Podlacha M, Hoinkis D, Rintz E, Brokowska J, Cyske Z, Wegrzyn G (2020) Underestimated aspect of mucopolysaccharidosis pathogenesis: global changes in cellular processes revealed by transcriptomic studies. Int J Mol Sci 21:1204. https://doi.org/10.3390/ijms21041204
Handschuh L (2019) Not only mutations matter: molecular picture of acute myeloid leukemia emerging from transcriptome studies. J Oncol 2019:7239206–7239236. https://doi.org/10.1155/2019/7239206
Kadamb R, Mittal S, Bansal N, Batra H, Saluja D (2013) Sin3: insight into its transcription regulatory functions. Eur J Cell Biol 92:237–246. https://doi.org/10.1016/j.ejcb.2013.09.001
Kelly TE (1976) The mucopolysaccharidoses and mucolipidoses. Clin Orthop Relat Res 114:116–133
Kobayashi H (2019) Recent trends in mucopolysaccharidosis research. J Hum Genet 64:127–137. https://doi.org/10.1038/s10038-018-0534-8
Kubaski F, de Oliveira PF, Michelin-Tirelli K, Graeff Burin M, Rojas-Málaga D, Brusius-Facchin AC, Leistner-Segal S, Giugliani R (2020) Diagnosis of mucopolysaccharidoses. Diagnostics 10:172. https://doi.org/10.3390/diagnostics10030172
Missaglia S, Coleman RA, Mordente A, Tavian D (2019) Neutral lipid storage diseases as cellular model to study lipid droplet function. Cells 8:187. https://doi.org/10.3390/cells8020187
Nag S, Larsson M, Robinson RC, Burtnick LD (2013) Gelsolin: the tail of a molecular gymnast. Cytoskeleton 70:360–384. https://doi.org/10.1002/cm.21117
Ortutay C, Nore BF, Vihinen M, Smith CI (2008) Phylogeny of Tec family kinases identification of a premetazoan origin of Btk, Bmx, Itk, Tec, Txk, and the Btk regulator SH3BP5. Adv Genet 64:51–80. https://doi.org/10.1016/S0065-2660(08)00803-1
Pierzynowska K, Gaffke L, Podlacha M, Węgrzyn G (2020) Genetic base of behavioral disorders in mucopolysaccharidoses: transcriptomic studies. Int J Mol Sci 21:1156. https://doi.org/10.3390/ijms21031156
Rintz E, Gaffke L, Podlacha M, Brokowska J, Cyske Z, Węgrzyn G, Pierzynowska K (2020) Transcriptomic changes related to cellular processes with particular emphasis on cell activation in lysosomal storage diseases from the group of mucopolysaccharidoses. Int J Mol Sci 21:3194. https://doi.org/10.3390/ijms21093194
Sorci-Thomas MG, Pollard RD, Thomas MJ (2015) What does procollagen C-endopeptidase enhancer protein 2 have to do with HDL-cholesteryl ester uptake? Or how I learned to stop worrying and love reverse cholesterol transport? Curr Opin Lipidol 26:420–425. https://doi.org/10.1097/MOL.0000000000000211
Sun A (2018) Lysosomal storage disease overview. Ann Transl Med 6:476. https://doi.org/10.21037/atm.2018.11.39
Valadares ER, Carneiro TB, Santos PM, Oliveira AC, Zabel B (2014) What is new in genetics and osteogenesis imperfecta classification? J Pediatr 90:536–541. https://doi.org/10.1016/j.jped.2014.05.003
Valenzuela I, Boronat S, Martínez-Sáez E, Clemente M, Sánchez-Montañez Á, Munell F, Carrascosa A, Macaya A (2017) Microcephaly with simplified gyral pattern, epilepsy and permanent neonatal diabetes syndrome (MEDS). A new patient and review of the literature. Eur J Med Genet 60:517–520. https://doi.org/10.1016/j.ejmg.2017.07.007
Yang YR, Kang DS, Lee C, Seok H, Follo MY, Cocco L, Suh PG (2016) Primary phospholipase C and brain disorders. Adv Biol Regul 61:80–85. https://doi.org/10.1016/j.jbior.2015.11.003
Zhou J, Lin J, Leung WT, Wang L (2020) A basic understanding of mucopolysaccharidosis: incidence, clinical features, diagnosis, and management. Intractable Rare Dis Res 9:1–9. https://doi.org/10.5582/irdr.2020.01011
Funding
This work was supported by National Science Center, Poland (project grant no. 2017/25/B/NZ2/00414).
Author information
Authors and Affiliations
Contributions
LG participated in planning the study, obtaining data and data analysis, KP contributed to planning the study, data analysis and interpretation, preparation of figures, and drafting the manuscripts, KK participated in data analysis, EP participated in planning the study and reviewing the manuscript, GW supervised the study, participated in data analysis, and drafted the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest is declared by the authors.
Ethics approval
All ethics-related documents belong to the Coriell Institute from which the cell lines were commercially purchased.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gaffke, L., Pierzynowska, K., Krzelowska, K. et al. Changes in expressions of genes involved in the regulation of cellular processes in mucopolysaccharidoses as assessed by fibroblast culture-based transcriptomic analyses. Metab Brain Dis 35, 1353–1360 (2020). https://doi.org/10.1007/s11011-020-00614-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-020-00614-2