Abstract
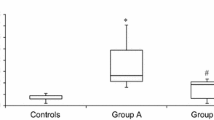
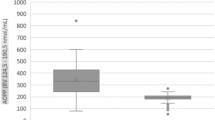
L-2-hydroxyglutaric aciduria (L2HGA) is an autosomal recessive disorder that is caused by deficiency of 2-hydroxyglutarate dehydrogenase. Pathophysiology of brain damage is poorly understood. In recent years, it was proposed that oxidative stress was elevated and led to brain injury. Aim of this study is to evaluate thiol/disulphide homeostasis as an indicator of oxidative stress in L2HGA patients who have been receiving antioxidant treatment. Sixteen L2HGA patients and 16 healthy individuals were included in the study. All the L2HGA patients were regularly followed up and presented neurological dysfunction at different grades. Fourteen patients had been receiving antioxidant treatment. Serum native thiol (-SH), total thiol (-SH + -S-S-) and disulphide (-S-S) levels were measured. Disulphide/native thiol, disulphide/total thiol and native thiol/total thiol ratios were calculated from these values. No significant difference was observed in -SH, -SH + -S-S-, -S-S levels between two groups. In addition to that, no increase of disulphide/native thiol and disulphide/total thiol ratios was detected. Thiol/disulphide homeostasis parameters were also compared between patients who had been receiving and not receiving antioxidant therapy; and between different types of antioxidant therapy and the results did not point to any significant difference. This is the first study that evaluates dynamic thiol/disulphide homeostasis as an indicator of oxidative stress in L2HGA and it has one of the largest sample sizes among previous studies. In our study we suggest that antioxidant therapy should be effective in preventing oxidative stress in L2HGA patients, which has been reported in previous studies and should be a part of standard therapy.
Similar content being viewed by others
References
Barschak AG, Sitta A, Deon M, de Oliveira MH, Haeser A, Dutra-Filho CS, Wajner M, Vargas CR (2006) Evidence that oxidative stress is increased in plasma from patients with maple syrup urine disease. Metab Brain Dis 21:279–286
Barth PG, Wanders RJ, Scholte HR et al (1998) L-2-hydroxyglutaric aciduria and lactic acidosis. J Inherit Metab Dis 21:251–254
Biswas S, Chida AS, Rahman I (2006) Redox modifications of protein-thiols: emerging roles in cell signaling. Biochem Pharmacol 71:551–564
Calabrese V, Lodi R, Tonon C, D'Agata V, Sapienza M, Scapagnini G, Mangiameli A, Pennisi G, Stella AMG, Butterfield DA (2005) Oxidative stress, mitochondrial dysfunction and cellular stress response in Friedreich's ataxia. J Neurol Sci 233:145–162
Chen E, Nyhan WL, Jakobs C, Greco CM, Barkovich AJ, Cox VA, Packman S (1996) L-2-Hydroxyglutaric aciduria: neuropathological correlations and first report of severe neurodegenerative disease and neonatal death. J Inherit Metab Dis 19:335–343
Cremers CM, Jakob U (2013) Oxidant sensing by reversible disulfide bond formation. J Biol Chem 288:26489–26496
da Rosa MS, João Ribeiro CA, Seminotti B, Teixeira Ribeiro R, Umpierrez Amaral A, de Moura Coelho D, de Oliveira FH, Leipnitz G, Wajner M (2015) In vivo intracerebral administration of L-2-hydroxyglutaric acid provokes oxidative stress and histopathological alterations in striatum and cerebellum of adolescent rats. Free Radic Biol Med 83:201–213. https://doi.org/10.1016/j.freeradbiomed.2015.02.008
da Silva CG, Bueno AR, Schuck PF et al (2003) L-2-hydroxyglutaric acid inhibits mitochondrial creatine kinase activity from cerebellum of developing rats. Int J Dev Neurosci 21:217–224
Deon M, Sitta A, Faverzani JL, Guerreiro GB, Donida B, Marchetti DP, Mescka CP, Ribas GS, Coitinho AS, Wajner M, Vargas CR (2015) Urinary biomarkers of oxidative stress and plasmatic inflammatory profile in phenylketonuric treated patients. Int J Dev Neurosci 47:259–265. https://doi.org/10.1016/j.ijdevneu.2015.10.001
Duran M, Kamerling JP, Bakker HD, van Gennip AH, Wadman SK (1980) L-2-Hydroxyglutaric aciduria: an inborn error of metabolism? J Inherit Metab Dis 3:109–112
Erel O, Neselioglu S (2014) A novel and automated assay for thiol/disulphide homeostasis. Clin Biochem 47:326–332. https://doi.org/10.1016/j.clinbiochem.2014.09.026
Fourati H, Ellouze E, Ahmadi M, Chaari D, Kamoun F, Hsairi I, Triki C, Mnif Z (2016) MRI features in 17 patients with l2 hydroxyglutaric aciduria. Eur J Radiol Open 3:245–250
Halliwell B (2006) Oxidative stress and neurodegeneration: where are we now? J Neurochem 97:1634–1658
Halliwell B, Gutteridge JMC (2007) Measurement of reactive species. In: Halliwell B, JMC G (eds) Free radicals in biology and medicine, 4th edn. Oxford University Press, Oxford, pp 268–340
Hoffmann GF, Kölker S (2016) Cerebral organic acid disorders and other disorders of lysine catabolism. In: Saudubray JM, Baumgartner MR, Walter J (eds) Inborn metabolic diseases: diagnosis and treatment, 6th edn. Springer-Verlag, Heidelberg, pp 333–348
Jellouli NK, Hadj Salem I, Ellouz E et al (2014) Founder effect confirmation of c.241A>G mutation in the L2HGDH gene and characterization of oxidative stress parameters in six Tunisian families with L-2-hydroxyglutaric aciduria. J Hum Genet 59:216–222. https://doi.org/10.1038/jhg.2014.4
Junqueira D, Brusque AM, Porciúncula LO, Rotta LN, Ribeiro CAJ, Frizzo MES, Filho CSD, Wannmacher CMD, Wyse ATS, Souza DO, Wajner M (2003) Effects of L-2-hydroxyglutaric acid on various parameters of the glutamatergic system in cerebral cortex of rats. Metab Brain Dis 18:233–243
Latini A, Scussiato K, Rosa RB et al (2003) Induction of oxidative stress by L-2-hydroxyglutaric acid in rat brain. J Neurosci Res 74:103–110
Lemineur T, Deby-Dupont G, Preiser JC (2006) Biomarkers of oxidative stress in critically ill patients: what should be measured, when and how? Curr Opin Clin Nutr Metab Care 9:704–710
Patay Z, Mills JC, Löbel U, Lambert A, Sablauer A, Ellison DW (2012) Cerebral neoplasms in L-2 hydroxyglutaric aciduria: 3 new cases and meta-analysis of literature data. AJNR Am J Neuroradiol 33:940–943. https://doi.org/10.3174/ajnr.A2869
Ribas GS, Manfredini V, de Marco MG, Vieira RB, Wayhs CY, Vanzin CS, Biancini GB, Wajner M, Vargas CR (2010a) Prevention by L-carnitine of DNA damage induced by propionic and L-methylmalonic acids in human peripheral leukocytes in vitro. Mutat Res 702:123–128. https://doi.org/10.1016/j.mrgentox.2010.07.008
Ribas GS, Manfredini V, de Mari JF, Wayhs CY, Vanzin CS, Biancini GB, Sitta A, Deon M, Wajner M, Vargas CR (2010b) Reduction of lipid and protein damage in patients with disorders of propionate metabolism under treatment: a possible protective role of L-carnitine supplementation. Int J Dev Neurosci 28:127–132. https://doi.org/10.1016/j.ijdevneu.2010.01.002
Ribeiro RT, Zanatta Â, Amaral AU, Leipnitz G, de Oliveira FH, Seminotti B, Wajner M (2018) Experimental evidence that in vivo intracerebral administration of L-2-Hydroxyglutaric acid to neonatal rats provokes disruption of redox status and histopathological abnormalities in the brain. Neurotox Res 33:681–692. https://doi.org/10.1007/s12640-018-9874-6
Rodrigues DGB, de Moura Coelho D, Sitta Â, Jacques CED, Hauschild T, Manfredini V, Bakkali A, Struys EA, Jakobs C, Wajner M, Vargas CR (2017) Experimental evidence of oxidative stress in patients with l-2-hydroxyglutaric aciduria and that l-carnitine attenuates in vitro DNA damage caused by d-2-hydroxyglutaric and l-2-hydroxyglutaric acids. Toxicol in Vitro 42:47–53. https://doi.org/10.1016/j.tiv.2017.04.006
Samuraki M, Komai K, Hasegawa Y, Kimura M, Yamaguchi S, Terada N, Yamada M (2008) A successfully treated adult patient with L-2-hydroxyglutaric aciduria. Neurology 70:1051–1052. https://doi.org/10.1212/01.wnl.0000287141.90944.95
Sauer SW, Opp S, Mahringer A, Kamiński MM, Thiel C, Okun JG, Fricker G, Morath MA, Kölker S (2010) Glutaric aciduria type I and methylmalonic aciduria: simulation of cerebral import and export of accumulating neurotoxic dicarboxylic acids in in vitro models of the blood-brain barrier and the choroid plexus. Biochim Biophys Acta 1802:552–560. https://doi.org/10.1016/j.bbadis.2010.03.003
Smeyne M, Smeyne RJ (2013) Glutathione metabolism and Parkinson's disease. Free Radic Biol Med 62:13–25. https://doi.org/10.1016/j.freeradbiomed.2013.05.001
Steele ML, Fuller S, Maczurek AE, Kersaitis C, Ooi L, Münch G (2013) Chronic inflammation alters production and release of glutathione and related thiols in human U373 astroglial cells. Cell Mol Neurobiol 33:19–30. https://doi.org/10.1007/s10571-012-9867-6
Steenweg ME, Salomons GS, Yapici Z, Uziel G, Scalais E, Zafeiriou DI, Ruiz-Falco ML, Mejaški-Bošnjak V, Augoustides-Savvopoulou P, Wajner M, Walter J, Verhoeven-Duif NM, Struys EA, Jakobs C, van der Knaap MS (2009) L-2-Hydroxyglutaric aciduria: pattern of MR imaging abnormalities in 56 patients. Radiology 251:856–865. https://doi.org/10.1148/radiol.2513080647
Steenweg ME, Jakobs C, Errami A, van Dooren SJM, Adeva Bartolomé MT, Aerssens P, Augoustides-Savvapoulou P, Baric I, Baumann M, Bonafé L, Chabrol B, Clarke JTR, Clayton P, Coker M, Cooper S, Falik-Zaccai T, Gorman M, Hahn A, Hasanoglu A, King MD, de Klerk HBC, Korman SH, Lee C, Meldgaard Lund A, Mejaški-Bošnjak V, Pascual-Castroviejo I, Raadhyaksha A, Rootwelt T, Roubertie A, Ruiz-Falco ML, Scalais E, Schimmel U, Seijo-Martinez M, Suri M, Sykut-Cegielska J, Trefz FK, Uziel G, Valayannopoulos V, Vianey-Saban C, Vlaho S, Vodopiutz J, Wajner M, Walter J, Walter-Derbort C, Yapici Z, Zafeiriou DI, Spreeuwenberg MD, Celli J, den Dunnen JT, van der Knaap MS, Salomons GS (2010) An overview of L-2-hydroxyglutarate dehydrogenase gene (L2HGDH) variants: a genotype-phenotype study. Hum Mutat 31:380–390. https://doi.org/10.1002/humu.21197
Topçu M, Jobard F, Halliez S, Coskun T, Yalçinkayal C, Gerceker FO, Wanders RJA, Prud'homme JF, Lathrop M, Özguc M, Fischer J (2004) L-2-Hydroxyglutaric aciduria: identification of a mutant gene C14orf160, localized on chromosome 14q22.1. Hum Mol Genet 13:2803–2811
Turell L, Radi R, Alvarez B (2013) The thiol pool in human plasma: the central contribution of albumin to redox processes. Free Radic Biol Med 65:244–253
Van Schaftingen E, Rzem R, Veiga-da-Cunha M (2009) L-2-Hydroxyglutaric aciduria, a disorder of metabolite repair. J Inherit Metab Dis 32:135–142. https://doi.org/10.1007/s10545-008-1042-3
Yilmaz K (2009) Riboflavin treatment in a case with l-2-hydroxyglutaric aciduria. Eur J Paediatr Neurol 13:57–60. https://doi.org/10.1016/j.ejpn.2008.01.003
Zubarioglu T, Kiykim E, Cansever MS, Neselioglu S, Aktuglu-Zeybek C, Erel O (2017) Evaluation of dynamic thiol/disulphide homeostasis as a novel indicator of oxidative stress in maple syrup urine disease patients under treatment. Metab Brain Dis 32:179–184. https://doi.org/10.1007/s11011-016-9898-7
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Mehmet Serif CANSEVER,Tanyel ZUBARIOGLU, Cigdem ORUC, Ertugrul KIYKIM, Alper GEZDIRICI, Salim NESELIOGLU, Ozcan EREL, Cengiz YALCINKAYA and Cigdem AKTUGLU-ZEYBEK declare that they have no conflict of interest.
The authors confirm independence from the sponsors; the content of the article has not been influenced by the sponsors.
All procedures followed were in accordance with the ethical standards of the local Ethical Committee of Cerrahpasa Medical faculty and with the Helsinki Declaration of 1975, as revised in 2000.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Take home message
Antioxidant therapy should prevent oxidative stress in L2HGA patients and should be a part of standard therapy.
Rights and permissions
About this article
Cite this article
Cansever, M.S., Zubarioglu, T., Oruc, C. et al. Oxidative stress among L-2-hydroxyglutaric aciduria disease patients: evaluation of dynamic thiol/disulfide homeostasis. Metab Brain Dis 34, 283–288 (2019). https://doi.org/10.1007/s11011-018-0354-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-018-0354-8