Abstract
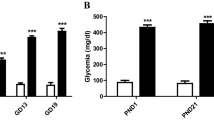
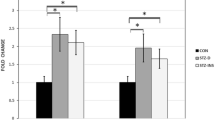
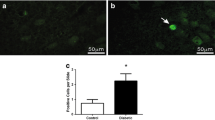
Diabetes during pregnancy impairs the development of the central nervous system (CNS) and causes cognitive and behavioral abnormalities in offspring. However, the exact mechanism by which the maternal diabetes affects the development of the brain remains to be elucidated. The aim of the present study was to investigate the effects of maternal diabetes in pregnancy on the expression of Bcl-2 and Bax genes and the numerical density of degenerating dark neurons (DNs) in the hippocampus of offspring at the first postnatal two weeks. Wistar female rats were maintained diabetic from a week before pregnancy through parturition and male offspring was sacrificed at P0, P7, and P14. Our findings demonstrated a significant down-regulation in the hippocampal expression of Bcl-2 in the diabetic group newborns (P < 0.05). In contrast, the mRNA expression of Bax was markedly up-regulated in the offspring born to diabetic dams at all of studied time-points (P < 0.05). Moreover, we found a striking increase in the numerical density of DNs in the various subfields of hippocampus of diabetic group pups (P < 0.05). The results of the present study revealed that maternal hyperglycemia during gestational period may result in disturbances in the expression of Bcl-2 and Bax genes as two important genes in neuronal apoptosis regulation and induces the production of DNs in the developing hippocampus of neonatal rats. These disturbances may be a reason for the cognitive, structural, and behavioral anomalies observed in offspring born to diabetic mothers. Furthermore, the control of maternal glycaemia by insulin administration in most cases normalized these negative impacts.


Similar content being viewed by others
References
Abe-Dohmae S, Harada N, Yamada K, Tanaka R (1993) Bcl-2 gene is highly expressed during neurogenesis in the central nervous system. Biochem Biophys Res Commun 191:915–921
Abusaad I, Mackay D, Zhao J, Stanford P, Collier DA, Everall IP (1999) Stereological estimation of the total number of neurons in the murine hippocampus using the optical disector. J Comp Neurol 408:560–566
Aerts L, Holemans K, Van Assche FA (1990) Maternal diabetes during pregnancy: consequences for the offspring. Diabetes Metab Rev 6:147–167
Ahmadpour SH, Haghir H (2011) Diabetes mellitus type 1 induces dark neuron formation in the dentate gyrus: a study by Gallyas' method and transmission electron microscopy. Romanian J Morphol Embryol 52:575–579
Ahmadpour S, Heravi MY (2012) Quantification of TUNEL assay in hippocampusof diabetic rats by MAT LAB: comparision with stereological method. J Clin Exp Pathol 2
Akhtar RS, Ness JM, Roth KA (2004) Bcl-2 family regulation of neuronal development and neurodegeneration. Biochim Biophys Acta 1644:189–203. doi:10.1016/j.bbamcr.2003.10.013
Allsopp TE, Wyatt S, Paterson HF, Davies AM (1993) The proto-oncogene bcl-2 can selectively rescue neurotrophic factor-dependent neurons from apoptosis. Cell 73:295–307
Alvarez EO, Beauquis J, Revsin Y, Banzan AM, Roig P, De Nicola AF, Saravia F (2009) Cognitive dysfunction and hippocampal changes in experimental type 1 diabetes. Behav Brain Res 198:224–230. doi:10.1016/j.bbr.2008.11.001
Babiker O (2007) Long-term effects of maternal diabetes on their offspring development and behaviours. Sudanese J Ped 8:133–146
Balu DT, Hodes GE, Hill TE, Ho N, Rahman Z, Bender CN, Ring RH, Dwyer JM, Rosenzweig-Lipson S, Hughes ZA et al (2009) Flow cytometric analysis of BrdU incorporation as a high-throughput method for measuring adult neurogenesis in the mouse. J Pharmacol Toxicol Methods 59:100–107. doi:10.1016/j.vascn.2008.12.002
Beauquis J, Roig P, Homo-Delarche F, De Nicola A, Saravia F (2006) Reduced hippocampal neurogenesis and number of hilar neurones in streptozotocin-induced diabetic mice: reversion by antidepressant treatment. Eur J Neurosci 23:1539–1546. doi:10.1111/j.1460-9568.2006.04691.x
Blázquez E, Velázquez E, Hurtado-Carneiro V, Ruiz-Albusac JM (2014) Insulin in the brain: its pathophysiological implications for states related with central insulin resistance, type 2 diabetes and Alzheimer's disease. Front Endocrinol 5:3389
Casson I, Clarke C, Howard C, McKendrick O, Pennycook S, Pharoah P, Platt M, Stanisstreet M, Van Velszen D, Walkinshaw S (1997) Outcomes of pregnancy in insulin dependent diabetic women: results of a five year population cohort study. BMJ 315:275–278
Chang T, Horal M, Jain S, Wang F, Patel R, Loeken M (2003) Oxidant regulation of gene expression and neural tube development: insights gained from diabetic pregnancy on molecular causes of neural tube defects. Diabetologia 46:538–545
Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR (2010) The BCL-2 family Reunion. Mol Cell 37:299–310
Colucci WS (1997) Molecular and cellular mechanisms of myocardial failure. Am J Cardiol 80:15L–25L
Dabelea D, Bennett PH, Pettitt DJ (2004) Long-term outcome of infants of diabetic mothers. Diabetes in women: adolescence, pregnancy and menopause. Lippincott Williams & Wilkins, Philadelphia, PA, pp 461–472
Deckwerth TL, Elliott JL, Knudson CM, Johnson EM Jr, Snider WD, Korsmeyer SJ (1996) BAX is required for neuronal death after trophic factor deprivation and during development. Neuron 17:401–411
Delascio Lopes C, Sinigaglia-Coimbra R, Mazzola J, Camano L, Mattar R (2011) Neurofunctional evaluation of young male offspring of rat dams with diabetes induced by streptozotocin. ISRN Endocrinol 2011:480656
Eidelman AI, Samueloff A (2002) The pathophysiology of the fetus of the diabetic mother. Proceedings of the Seminars in perinatology. Elsevier
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516
Eriksson U, Dahlström E, Larsson KS, Hellerström C (1982) Increased incidence of congenital malformations in the offspring of diabetic rats and their prevention by maternal insulin therapy. Diabetes 31:1–6
Eriksson U, Bone A, Turnbull D, Baird J (1989) Timed interruption of insulin therapy in diabetic BB/E rat pregnancy: effect on maternal metabolism and fetal outcome. Acta Endocrinol 120:800–810
Farlie PG, Dringen R, Rees SM, Kannourakis G, Bernard O (1995) Bcl-2 transgene expression can protect neurons against developmental and induced cell death. Proc Natl Acad Sci U S A 92:4397–4401
Fayard E, Xue G, Parcellier A, Bozulic L, Hemmings BA (2010) Protein kinase B (PKB/Akt), a key mediator of the PI3K signaling pathway. Curr Top Microbiol Immunol 346:31–56. doi:10.1007/82_2010_58
Fetita L-S, Sobngwi E, Serradas P, Calvo F, Gautier J-F (2006) Consequences of fetal exposure to maternal diabetes in offspring. J Clin Endocrinol Metab 91:3718–3724
Foghi K, Ahmadpour S (2014) Diabetes mellitus Type1 and neuronal degeneration in ventral and dorsal hippocampus. Iran J Pathol 9:33–37
Gabbay-Benziv R, Reece EA, Wang F, Yang P (2015) Birth defects in pregestational diabetes: defect range, glycemic threshold and pathogenesis. World J Diabetes 6:481
Galetic I, Andjelkovic M, Meier R, Brodbeck D, Park J, Hemmings BA (1999) Mechanism of protein kinase B activation by insulin/insulin-like growth factor-1 revealed by specific inhibitors of phosphoinositide 3-kinase--significance for diabetes and cancer. Pharmacol Ther 82:409–425
Gallyas F, Zoltay G, Balas I (1992) An immediate light microscopic response of neuronal somata, dendrites and axons to contusing concussive head injury in the rat. Acta Neuropathol 83:394–401
Garcia I, Martinou I, Tsujimoto Y, Martinou JC (1992) Prevention of programmed cell death of sympathetic neurons by the bcl-2 proto-oncogene. Science 258:302–304
Ge X, Shi Y, Li J, Zhang Z, Lin X, Zhan J, Ge H, Xu J, Yu Q, Leng Y (2015) Development of the human fetal hippocampal formation during early second trimester. NeuroImage 119:33–43
Gerfen C, Paxinos G (2004) The rat nervous system. Academic Press, San Diego
Ghafari S, Golalipour E, Golalipour MJ, Ghafari S, Golalipour E, Golalipour M (2015) Alterations of the Giant pyramidal neurons (Betz cells) in brain cortex of rat offspring born from gestational diabetic dams: a morphometric study. Int J Morphol 33:1120–1125
Golalipour M, Kafshgiri SK, Ghafari S (2012) Gestational diabetes induced neuronal loss in CA1 and CA3 subfields of rat hippocampus in early postnatal life. Folia Morphol (Warsz) 71:71–77
Gray M, Shirasaki DI, Cepeda C, André VM, Wilburn B, Lu X-H, Tao J, Yamazaki I, Li S-H, Sun YE (2008) Full-length human mutant huntingtin with a stable polyglutamine repeat can elicit progressive and selective neuropathogenesis in BACHD mice. J Neurosci 28:6182–6195
Gundersen H, Bagger P, Bendtsen T, Evans S, Korbo L, Marcussen N, Møller A, Nielsen K, Nyengaard J, Pakkenberg B (1988a) The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS 96:857–881
Gundersen H, Bendtsen TF, Korbo L, Marcussen N, Møller A, Nielsen K, Nyengaard J, Pakkenberg B, Sørensen FB, Vesterby A (1988b) Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS 96:379–394
Haghir H, Sankian M, Kheradmand H, Hami J (2013) The effects of induced type-I diabetes on developmental regulation of insulin & insulin like growth factor-1 (IGF-1) receptors in the cerebellum of rat neonates. Metab Brain Dis 28:397–410
Hami J, Sadr-Nabavi A, Sankian M, Haghir H (2012) Sex differences and left-right asymmetries in expression of insulin and insulin-like growth factor-1 receptors in developing rat hippocampus. Brain Struct Funct 217:293–302. doi:10.1007/s00429-011-0358-1
Hami J, Sadr-Nabavi A, Sankian M, Balali-Mood M, Haghir H (2013) The effects of maternal diabetes on expression of insulin-like growth factor-1 and insulin receptors in male developing rat hippocampus. Brain Struct Funct 218:73–84
Hami J, Karimi R, Haghir H, Gholamin M, Sadr-Nabavi A (2015a) Diabetes in pregnancy adversely affects the expression of glycogen synthase kinase-3β in the hippocampus of rat neonates. J Mol Neurosci 57:273–281
Hami J, Kerachian M-A, Karimi R, Haghir H, Sadr-Nabavi A (2015b) Effects of streptozotocin-induced type 1 maternal diabetes on PI3K/AKT signaling pathway in the hippocampus of rat neonates. J Recept Signal Transduct 36:1–7
Hami J, Shojae F, Vafaee-Nezhad S, Lotfi N, Kheradmand H, Haghir H (2015c) Some of the experimental and clinical aspects of the effects of the maternal diabetes on developing hippocampus. World J Diabetes 6:412
Hami J, Vafaei-Nezhad S, Haghir D, Haghir H (2015d) Insulin-like growth factor-1 receptor is differentially distributed in developing Cerebellar cortex of rats born to diabetic mothers. J Mol Neurosci 1–12
Hami J, Vafaei-nezhad S, Ghaemi K, Sadeghi A, Ivar G, Shojae F, Hosseini M (2016) Stereological study of the effects of maternal diabetes on cerebellar cortex development in rat. Metab Brain Dis 1–10
Hicks R, Soares H, Smith D, McIntosh T (1996) Temporal and spatial characterization of neuronal injury following lateral fluid-percussion brain injury in the rat. Acta Neuropathol 91:236–246
Ho N, Sommers MS, Lucki I (2013) Effects of diabetes on hippocampal neurogenesis: links to cognition and depression. Neurosci Biobehav Rev 37:1346–1362. doi:10.1016/j.neubiorev.2013.03.010
Howard V, Reed M 2004 Unbiased stereology: three-dimensional measurement in microscopy. Garland Science
Humphrey T (1967) The development of the human hippocampal fissure. J Anat 101:655
Hung RWY, Chow AW (1997) Apoptosis: molecular mechanisms, regulation and role in pathogenesis. Can J Infect Dis 8:103
Ishida K, Shimizu H, Hida H, Urakawa S, Ida K, Nishino H (2004) Argyrophilic dark neurons represent various states of neuronal damage in brain insults: some come to die and others survive. Neuroscience 125:633–644
Jafari Anarkooli I, Sankian M, Ahmadpour S, Varasteh A-R, Haghir H (2008) Evaluation of Bcl-2 family gene expression and Caspase-3 activity in hippocampus STZ-induced diabetic rats. Exp Diabetes Res 2008
Jawerbaum A, White V (2010) Animal models in diabetes and pregnancy. Endocr Rev 31:680–701
Johnson GA, Calabrese E, Badea A, Paxinos G, Watson C (2012) A multidimensional magnetic resonance histology atlas of the Wistar rat brain. NeuroImage 62:1848–1856
Johnston MV, Trescher WH, Ishida A, Nakajima W (2001) Neurobiology of hypoxic-ischemic injury in the developing brain. Pediatr Res 49:735–741. doi:10.1203/00006450-200106000-00003
Kafshgiri SK, Ghafari S, Golalipour MJ (2014) Gestational diabetes induces neuronal loss in dentate gyrus in rat offspring. J Neurol Sci 31:316–324
Kasznicki J, Kosmalski M, Sliwinska A, Mrowicka M, Stanczyk M, Majsterek I, Drzewoski J (2012) Evaluation of oxidative stress markers in pathogenesis of diabetic neuropathy. Mol Biol Rep 39:8669–8678
Khaksar Z, Jelodar G, Hematian H (2011) Morphometric study of cerebrum in fetuses of diabetic mothers. Iran J Vet Res 12:199–204
Kherani ZS, Auer RN (2008) Pharmacologic analysis of the mechanism of dark neuron production in cerebral cortex. Acta Neuropathol 116:447–452
Korsmeyer SJ (1999) BCL-2 gene family and the regulation of programmed cell death. Cancer Res 59:1693s–1700s
Krajewski S, Krajewska M, Shabaik A, Miyashita T, Wang HG, Reed JC (1994) Immunohistochemical determination of in vivo distribution of Bax, a dominant inhibitor of bcl-2. Am J Pathol Dec 145:1323–1336
Lapolla A, Dalfra M, Fedele D (2005) Insulin therapy in pregnancy complicated by diabetes: are insulin analogs a new tool? Diabetes Metab Res Rev 21:241–252
Lappas M, Hiden U, Desoye G, Froehlich J, Hauguel-de Mouzon S, Jawerbaum A (2011) The role of oxidative stress in the pathophysiology of gestational diabetes mellitus. Antioxid Redox Signal 15:3061–3100. doi:10.1089/ars.2010.3765
Lewis S (2012) Learning and memory: hippocampus plays multiple choice. Nat Rev Neurosci 13:600
Li ZG, Zhang W, Grunberger G, Sima AA (2002) Hippocampal neuronal apoptosis in type 1 diabetes. Brain Res 946:221–231
Linder K, Schleger F, Kiefer-Schmidt I, Fritsche L, Kümmel S, Heni M, Weiss M, Häring H-U, Preissl H, Fritsche A (2015) Gestational diabetes impairs human fetal postprandial brain activity. J Clin Endocrinol Metab 100:4029–4036
Lotfi N, Hami J, Hosseini M, Haghir D, Haghir H (2016) Diabetes during pregnancy enhanced neuronal death in the hippocampus of rat offspring. Int J Dev Neurosci 51:28–35. doi:10.1016/j.ijdevneu.2016.04.009
Lynch CP, Baker N, Korte JE, Mauldin JG, Mayorga ME, Hunt KJ (2015) Increasing prevalence of diabetes during pregnancy in South Carolina. J Women's Health 24:316–323
Martinou JC, Dubois-Dauphin M, Staple JK, Rodriguez I, Frankowski H, Missotten M, Albertini P, Talabot D, Catsicas S, Pietra C et al (1994) Overexpression of BCL-2 in transgenic mice protects neurons from naturally occurring cell death and experimental ischemia. Neuron 13:1017–1030
McQuillen PS, Sheldon RA, Shatz CJ, Ferriero DM (2003) Selective vulnerability of subplate neurons after early neonatal hypoxia-ischemia. J Neurosci 23:3308–3315
Merry DE, Veis DJ, Hickey WF, Korsmeyer SJ (1994) Bcl-2 protein expression is widespread in the developing nervous system and retained in the adult PNS. Development 120:301–311
Meur S, Mann NP (2007) Infant outcomes following diabetic pregnancies. Paediatr Child Health 17:217–222
Mirarab Razi E, Ghafari S, Golalipour MJ (2015) Effect of gestational diabetes on purkinje and granule cells distribution of the rat cerebellum in 21 and 28 days of postnatal life. Basic Clin Neurosci 6:6–13
Moran C, Phan TG, Chen J, Blizzard L, Beare R, Venn A, Munch G, Wood AG, Forbes J, Greenaway TM et al (2013) Brain atrophy in type 2 diabetes: regional distribution and influence on cognition. Diabetes Care 36:4036–4042. doi:10.2337/dc13-0143
Mouton PR 2002 Principles and practices of unbiased stereology: an introduction for bioscientists. JHU Press
Nelson CA, Wewerka S, Thomas KM, deRegnier R-a, Tribbey-Walbridge S, Georgieff M (2000) Neurocognitive sequelae of infants of diabetic mothers. Behav Neurosci 114:950
Nelson TJ, Sun M-K, Hongpaisan J, Alkon DL (2008) Insulin, PKC signaling pathways and synaptic remodeling during memory storage and neuronal repair. Eur J Pharmacol 585:76–87
Nolan CJ, Damm P, Prentki M (2011) Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet 378:169–181
Nomura Y, Marks DJ, Grossman B, Yoon M, Loudon H, Stone J, Halperin JM (2012) Exposure to gestational diabetes mellitus and low socioeconomic status: effects on neurocognitive development and risk of attention-deficit/hyperactivity disorder in offspring. Arch Pediatr Adolesc Med 166:337–343
Oltvai ZN, Milliman CL, Korsmeyer SJ (1993) Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74:609–619
Ooigawa H, Nawashiro H, Fukui S, Otani N, Osumi A, Toyooka T, Shima K (2006) The fate of Nissl-stained dark neurons following traumatic brain injury in rats: difference between neocortex and hippocampus regarding survival rate. Acta Neuropathol 112:471–481
Ornoy A (2005) Growth and neurodevelopmental outcome of children born to mothers with pregestational and gestational diabetes. Pediatr Endocrinol Rev PER.3:104–113
Ornoy A (2011) Prenatal origin of obesity and their complications: gestational diabetes, maternal overweight and the paradoxical effects of fetal growth restriction and macrosomia. Reprod Toxicol 32:205–212
Ornoy A, Ratzon N, Greenbaum C, Peretz E, Soriano D, Dulitzky M (1998) Neurobehaviour of school age children born to diabetic mothers. Arch Dis Child-Fetal Neonatal Ed 79:F94–F99
Ornoy A, Ratzon N, Greenbaum C, Wolf A, Dulitzky M (2001) School-age children born to diabetic mothers and to mothers with gestational diabetes exhibit a high rate of inattention and fine and gross motor impairment. J Pediatr Endocrinol Metab 14:681–690
Pamidi N, Satheesha NB (2011) Effect of streptozotocin induced diabetes on rat hippocampus. Bratisl Lek Listy 113:583–588
Panel IC (2010) International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 33:676–682
Persaud OD (2007) Maternal diabetes and the consequences for her offspring. J Develop Disab 1:101–134
Petersen MB, Pedersen SA, Greisen G, Pedersen JF, Mølsted-Pedersen L (1988) Early growth delay in diabetic pregnancy: relation to psychomotor development at age 4. Br Med J (Clin Res Ed) 296:598
Pfaff WW, Howard RJ, Patton PR, Adams VR, Rosen CB, Reed AI (1998) Delayed graft function after renal transplantation. Transplantation 65:219–223
Plum L, Schubert M, Brüning JC (2005) The role of insulin receptor signaling in the brain. Trends Endocrinol Metab 16:59–65
Popken GJ, Hodge RD, Ye P, Zhang J, Ng W, O'Kusky JR, D'Ercole AJ (2004) In vivo effects of insulin-like growth factor-I (IGF-I) on prenatal and early postnatal development of the central nervous system. Eur J Neurosci 19:2056–2068
Ratzon N, Greenbaum C, Dulitzky M, Ornoy A (2000) Comparison of the motor development of school-age children born to mothers with and without diabetes mellitus. Phys Occup Ther Pediatr 20:43–57
Ray J, O’Brien T, Chan W (2002) Preconception care and the risk of congenital anomalies in the offspring of women with diabetes mellitus: a meta-analysis. Obstet Gynecol Surv 57:71–72
Razi EM, Ghafari S, Hojati V, Golalipour MJ, Razi E, Ghafari S, Hojati V, Golalipour M (2014) Effect of gestational diabetes on neuronal cells in rat cerebellum in early postnatal life. Int J Morphol 32:420–425
Reece EA (2010) The fetal and maternal consequences of gestational diabetes mellitus. J Maternal-Fetal Neonatal Med 23:199–203
Retnakaran R, Shah BR (2015) Fetal sex and the natural history of maternal risk of diabetes during and after pregnancy. J Clin Endocrinol Metab 100:2574–2580
Revsin Y, Rekers NV, Louwe MC, Saravia FE, De Nicola AF, de Kloet ER, Oitzl MS (2009) Glucocorticoid receptor blockade normalizes hippocampal alterations and cognitive impairment in streptozotocin-induced type 1 diabetes mice. Neuropsychopharmacology 34:747–758. doi:10.1038/npp.2008.136
Rice D, Barone S Jr (2000) Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect 108:511
Rizzo T, Metzger BE, Burns WJ, Burns K (1991) Correlations between antepartum maternal metabolism and intelligence of offspring. N Engl J Med 325:911–916
Rizzo TA, Dooley SL, Metzger BE, Cho NH, Ogata ES, Silverman BL (1995) Prenatal and perinatal influences on long-term psychomotor development in offspring of diabetic mothers. Am J Obstet Gynecol 173:1753–1758
Roberts RO, Knopman DS, Przybelski SA, Mielke MM, Kantarci K, Preboske GM, Senjem ML, Pankratz VS, Geda YE, Boeve BF et al (2014) Association of type 2 diabetes with brain atrophy and cognitive impairment. Neurology 82:1132–1141. doi:10.1212/WNL.0000000000000269
Russell JW, Sullivan KA, Windebank AJ, Herrmann DN, Feldman EL (1999) Neurons undergo apoptosis in animal and cell culture models of diabetes. Neurobiol Dis 6:347–363. doi:10.1006/nbdi.1999.0254
Sadeghi A, Hami J, Razavi S, Esfandiary E, Hejazi Z (2016) The effect of diabetes mellitus on apoptosis in hippocampus: cellular and molecular aspects. Int J Prev Med 7:57. doi:10.4103/2008-7802.178531
Sadeghian H, Jafarian M, Karimzadeh F, Kafami L, Kazemi H, Coulon P, Ghabaee M, Gorji A (2012) Neuronal death by repetitive cortical spreading depression in juvenile rat brain. Exp Neurol 233:438–446
Salvesen DR, Freeman J, Brudenell JM, Nicolaides KH (1993) Prediction of fetal acidaemia in pregnancies complicated by maternal diabetes mellitus by biophysical profile scoring and fetal heart rate monitoring. BJOG: Int J Obstet Gynaecol 100:227–233
Saravia FE, Beauquis J, Revsin Y, Homo-Delarche F, de Kloet ER, De Nicola AF (2006) Hippocampal neuropathology of diabetes mellitus is relieved by estrogen treatment. Cell Mol Neurobiol 26:943–957. doi:10.1007/s10571-006-9096-y
Sato N, Morishita R (2014) Brain alterations and clinical symptoms of dementia in diabetes: abeta/tau-dependent and independent mechanisms. Front Endocrinol (Lausanne) 5:143. doi:10.3389/fendo.2014.00143
Sato T, Hanada M, Bodrug S, Irie S, Iwama N, Boise LH, Thompson CB, Golemis E, Fong L, Wang HG et al (1994) Interactions among members of the bcl-2 protein family analyzed with a yeast two-hybrid system. Proc Natl Acad Sci U S A 91:9238–9242
Schapiro AC, Turk-Browne NB, Norman KA, Botvinick MM (2016) Statistical learning of temporal community structure in the hippocampus. Hippocampus 26:3–8
Scherle W (1970) A simple method for volumetry of organs in quantitative stereology. Mikroskopie 26:57
Schwartz R, Teramo KA (2000) Effects of diabetic pregnancy on the fetus and newborn. Proceedings of the Seminars in perinatology. Elsevier
Schwartz R, Gruppuso PA, Petzold K, Brambilla D, Hiilesmaa V, Teramo KA (1994) Hyperinsulinemia and macrosomia in the fetus of the diabetic mother. Diabetes Care 17:640–648
Sedlak TW, Oltvai ZN, Yang E, Wang K, Boise LH, Thompson CB, Korsmeyer SJ (1995) Multiple bcl-2 family members demonstrate selective dimerizations with Bax. Proc Natl Acad Sci U S A 92:7834–7838
Shin B-C, Fujikura K, Suzuki T, Tanaka S, Takata K (1997) Glucose transporter GLUT3 in the rat placental barrier: a possible machinery for the transplacental transfer of glucose. Endocrinology 138:3997–4004
Singh BS, Westfall TC, Devaskar SU (1997) Maternal diabetes-induced hyperglycemia and acute intracerebral Hyperinsulinism suppress fetal brain neuropeptide Y concentrations 1. Endocrinology 138:963–969
Squire LR (1992) Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev 99:195
Sterio D (1984) The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc 134:127–136
Styrud J, Thunberg L, Nybacka O, Eriksson U (1995) Correlations between maternal metabolism and deranged development in the offspring of normal and diabetic rats. Pediatr Res 37:343–353
Suvisaari JM, Taxell-Lassas V, Pankakoski M, Haukka JK, Lönnqvist JK, Häkkinen LT (2013) Obstetric complications as risk factors for schizophrenia spectrum psychoses in offspring of mothers with psychotic disorder. Schizophr Bull 39:1056–1066
Takata K, Fujikura K, Shin B-C (1997) Ultrastructure of the rodent placental labyrinth: a site of barrier and transport. J Reprod Dev 43:13–24
Tan S-E (2009) Activation of hippocampal nitric oxide and calcium/calmodulin-dependent protein kinase II in response to Morris water maze learning in rats. Pharmacol Biochem Behav 92:260–266
Tehranipour M, Khakzad M (2008) Effect of maternal diabetes on hippocampus neuronal density in neonatal rats. J Biol Sci 6:1027–1032
Tennant PW, Bilous RW, Prathapan S, Bell R (2015) Risk and recurrence of serious adverse outcomes in the first and second pregnancies of women with preexisting diabetes. Diabetes Care 38:610–619
ter Braak EW, Evers IM, Willem Erkelens D, Visser GH (2002) Maternal hypoglycemia during pregnancy in type 1 diabetes: maternal and fetal consequences. Diabetes Metab Res Rev 18:96–105
Todorova K, Mazneikova V, Ivanov S, Genova M (2005) The frequency of mild and severe fetal malformations in diabetic women with high values of glycosilated hemoglobin in early pregnancy. Akush Ginekol (Sofiia) 44:3–10
Towner D, Kjos SL, Leung B, Montoro MM, Xiang A, Mestman JH, Buchanan TA (1995) Congenital malformations in pregnancies complicated by NIDDM: increased risk from poor maternal metabolic control but not from exposure to sulfonylurea drugs. Diabetes Care 18:1446–1451
Turgut YB, Turgut M (2011) A mysterious term hippocampus involved in learning and memory. Childs Nerv Syst 27:2023–2025
Vafaei-Nezhad S, Hami J, Sadeghi A, Ghaemi K, Hosseini M, Abedini MR, Haghir H (2016) The impacts of diabetes in pregnancy on hippocampal synaptogenesis in rat neonates. Neuroscience
Vincent AM, Russell JW, Low P, Feldman EL (2004) Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev 25:612–628
Wender-Ozegowska E, Wroblewska K, Zawiejska A, Pietryga M, Szczapa J, Biczysko R (2005) Threshold values of maternal blood glucose in early diabetic pregnancy--prediction of fetal malformations. Acta Obstet Gynecol Scand 84:17–25. doi:10.1111/j.0001-6349.2005.00606.x
White FA, Keller-Peck CR, Knudson CM, Korsmeyer SJ, Snider WD (1998) Widespread elimination of naturally occurring neuronal death in Bax-deficient mice. J Neurosci 18:1428–1439
Wilhelm J, Vytasek R, Uhlik J, Vajner L (2016) Oxidative stress in the developing rat brain due to production of reactive oxygen and nitrogen species. Oxidative Med Cell Longev 2016:5057610. doi:10.1155/2016/5057610
Yao R, Cooper GM (1995) Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth factor. Science 267:2003–2006
Yonguc GN, Dodurga Y, Adiguzel E, Gundogdu G, Kucukatay V, Ozbal S, Yilmaz I, Cankurt U, Yilmaz Y, Akdogan I (2015) Grape seed extract has superior beneficial effects than vitamin E on oxidative stress and apoptosis in the hippocampus of streptozotocin induced diabetic rats. Gene 555:119–126
Yuan J, Horvitz HR (2004) A first insight into the molecular mechanisms of apoptosis. Cell 116:S53–S56
Zhang WJ, Tan YF, Yue JT, Vranic M, Wojtowicz JM (2008) Impairment of hippocampal neurogenesis in streptozotocin-treated diabetic rats. Acta Neurol Scand Mar 117:205–210. doi:10.1111/j.1600-0404.2007.00928.x
Zhao C-H, Liu H-Q, Cao R, Ji A-L, Zhang L, Wang F, Yang R-H (2012) Effects of dietary fish oil on learning function and apoptosis of hippocampal pyramidal neurons in streptozotocin-diabetic rats. Brain Res 1457:33–43
Zhou J, Wang L, Ling S, Zhang X (2007) Expression changes of growth-associated protein-43 (GAP-43) and mitogen-activated protein kinase phosphatase-1 (MKP-1) and in hippocampus of streptozotocin-induced diabetic cognitive impairment rats. Exp Neurol 206:201–208
Zsombok A, Tóth Z, Gallyas F (2005) Basophilia, acidophilia and argyrophilia of “dark”(compacted) neurons during their formation, recovery or death in an otherwise undamaged environment. J Neurosci Methods 142:145–152
Acknowledgements
This study was financially supported by Mashhad University of Medical Sciences (MUMS) Grant (Nr: 940677). The authors gratefully thank Dr. Akram Sadeghi, Mr. Saeed Vafaei-Nezhad, and Mr. Ghasem Ivar for their technical assistance and helpful comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial or nonfinancial conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Haghir, H., Hami, J., Lotfi, N. et al. Expression of apoptosis-regulatory genes in the hippocampus of rat neonates born to mothers with diabetes. Metab Brain Dis 32, 617–628 (2017). https://doi.org/10.1007/s11011-017-9950-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-017-9950-2