Abstract
To investigate alterations of functional connectivity density (FCD) in patients with end-stage renal disease (ESRD) by using resting-state functional magnetic resonance imaging (rs-fMRI). Medical research ethics committee approval from Jinling hospital and written informed consent from each subject were obtained. Forty six patients with ESRD, consisting of 21 patients minimal nephrotic encephalopathy (MNE) and 25 non-nephro-encephalopathy (non-NE), as well as 23 healthy controls underwent rs-fMRI. Neuropsychological tests were performed in all subjects, while laboratory tests were performed in ESRD patients. A voxel-wise whole brain functional connectivity analysis was used to generate long- and short-range FCD maps. The maps among MNE, non-NE, and healthy controls groups were compared by using one-way analysis of variance tests. A multiple regression analysis was performed to evaluate the correlations between FCD and the variables of neuropsychological or laboratory tests. Compared with healthy controls, non-NE showed decreased long-range FCD mainly in parietal lobe. Moreover, MNE showed further decreased long-range FCD in bilateral middle prefrontal cortex (MPFC), anterior cingulate cortex (ACC) and right superior frontal gyrus. Meanwhile, non-NE showed decreased short-range FCD mainly in frontal cortex, and further reduction in bilateral ACC and right superior parietal gyrus in MNE. In addition, patients with ESRD mainly exhibited increased long-range FCD in left temporal lobe and caudate; and increased short-range FCD in bilateral orbitofrontal cortex and temporal gyri (P < 0.05, AlphaSim corrected). The number connection test type A score, serum creatinine, urea, and dialysis duration showed negative correlation with FCD in some brain regions, while the digital symbol test scores positively correlated with short-range FCD in left inferior parietal lobule (all P < 0.05, AlphaSim corrected). The prominent long- and short-range FCD reduction was found mainly in default mode network (DMN) and bilateral frontal and parietal lobes, while the progressively decreased long- and short-range FCD in ACC/MPFC and the long-range FCD in left superior frontal gyrus from non-NE to MNE was associated with cognition dysfunction in ESRD patients.




Similar content being viewed by others
Abbreviations
- ESRD:
-
End-stage renal disease
- FCD:
-
Functional connectivity density
- rs-fMRI:
-
Resting-state functional magnetic resonance imaging
- DMN:
-
Default mode network
- MNE:
-
Minimal nephro-encephalopathy
- non-NE:
-
Patients without nephro-encephalopathy
- HCs:
-
Healthy controls
- BA:
-
Brodmann’s area
- MNI:
-
Montreal neurological institute
- TPO:
-
Temporal pole
- SPG:
-
Superior parietal gyrus
- IPL:
-
Inferior parietal lobule
- PCu:
-
Precuneus
- PCC:
-
Posterior cingulate cortex
- ITG:
-
Inferior temporal gyrus
- ORBsup:
-
Orbitofrontal cortex (superior)
- ORBmid:
-
Orbitofrontal cortex (middle)
- ACC:
-
Anterior cingulate cortex
- SFGmed:
-
Superior frontal gyrus (medial)
- MFG:
-
Middle frontal gyrus
- SFG:
-
Superior frontal gyrus
- STG:
-
Superior temporal gyrus
References
Anticevic A, Cole MW, Murray JD, Corlett PR, Wang XJ, Krystal JH (2012) The role of default network deactivation in cognition and disease. Trends Cogn Sci 16(12):584–592
Bajaj JS, Wade JB, Sanyal AJ (2009) Spectrum of neurocognitive impairment in cirrhosis: implications for the assessment of hepatic encephalopathy. Hepatology 50(6):2014–2021
Beucke JC, Sepulcre J, Talukdar T, et al (2013) Abnormally high degree connectivity of the orbitofrontal cortex in obsessive-compulsive disorder. JAMA Psychiatry 70(6):619–629
Brouns R, De Deyn PP (2004) Neurological complications in renal failure: a review. Clin Neurol Neurosurg 107(1):1–16
Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJ (2009) Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev 33(3):279–296
Buckner RL (2010) Human functional connectivity: new tools, unresolved questions. Proc Natl Acad Sci U S A 107(24):10769–10770
Buckner RL, Andrews-Hanna JR, Schacter DL (2008) The brain's default network. Ann N Y Acad Sci 1124(1):1–38
Buckner RL, Sepulcre J, Talukdar T, et al (2009) Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci 29(6):1860–1873
Bugnicourt JM, Godefroy O, Chillon JM, Choukroun G, Massy ZA (2013) Cognitive disorders and dementia in CKD: the neglected kidney-brain axis. J Am Soc Nephrol 24(3):353–363
Bullmore E, Sporns O (2009) Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10(3):186–198
Chao-Gan Y, Yu-Feng Z (2010) DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front Syst Neurosci 4:13
Chen HJ, Zhu XQ, Shu H, et al (2012) Structural and functional cerebral impairments in cirrhotic patients with a history of overt hepatic encephalopathy. Eur J Radiol 81(10):2463–2469
Fazekas G, Fazekas F, Schmidt R, et al (1996) Pattern of cerebral blood flow and cognition in patients undergoing chronic haemodialysis treatment. Nucl Med Commun 17(7):603–608
Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT (2002) Hepatic encephalopathy–definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology 35(3):716–721
Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005) The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 102(27):9673–9678
Geissler A, Fründ R, Kohler S, Eichhorn HM, Krämer BK, Feuerbach S (1995) Cerebral metabolite patterns in dialysis patients: evaluation with H-1 MR spectroscopy. Radiology 194(3):693–697
Giang LM, Weiner DE, Agganis BT, et al (2010) Cognitive function and dialysis adequacy: no clear relationship. Am J Nephrol 33(1):33–38
Grant I (2009) Neuropsychological assessment of neuropsychiatric and neuromedical disorders. Oxford University Press
Harciarek M, Williamson JB, Biedunkiewicz B, Lichodziejewska-Niemierko M, Dębska-Ślizień A, Rutkowski B, et al (2012) Risk factors for selective cognitive decline in dialyzed patients with end-stage renal disease: evidence from verbal fluency analysis. J Int Neuropsychol Soc 18(1):162–167
Hirakata H, Yao H, Osato S, et al (1992) CBF and oxygen metabolism in hemodialysis patients: effects of anemia correction with recombinant human EPO. Am J Phys 262(5 Pt 2):F737–F743
Kanai H, Hirakata H, Nakane H, et al (2001) Depressed cerebral oxygen metabolism in patients with chronic renal failure: a positron emission tomography study. Am J Kidney Dis 38(4 Suppl 1):S129–S133
Kong X, Wen JQ, Qi RF, et al (2014) Diffuse interstitial brain edema in patients with end-stage renal disease undergoing hemodialysis: a tract-based spatial statistics study. Medicine (Baltimore) 93(28):e313
Krishnan AV, Kiernan MC (2009) Neurological complications of chronic kidney disease. Nat Rev Neurol 5(10):542–551
Kurella Tamura M, Yaffe K (2010) Dementia and cognitive impairment in ESRD: diagnostic and therapeutic strategies. Kidney Int 79(1):14–22
Li C, Su HH, Qiu YW, et al (2014) Regional homogeneity changes in hemodialysis patients with end stage renal disease: in vivo resting-state functional MRI study. PLoS One 9(2):e87114
Liang X, Wen J, Ni L et al (2013) Altered pattern of spontaneous brain activity in the patients with end-stage renal disease: a resting-state functional MRI study with regional homogeneity analysis. PLoS One 8(8):e71507
McQuillan R, Jassal SV (2010) Neuropsychiatric complications of chronic kidney disease. Nat Rev Nephrol 6(8):471–479
Menon V, Uddin LQ (2010) Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 214(5–6):655–667
Murray AM, Knopman DS (2010) Cognitive impairment in CKD: no longer an occult burden. Am J Kidney Dis 56(4):615–618
Ni L, Wen J, Zhang LJ, et al (2014) Aberrant default-mode functional connectivity in patients with end-stage renal disease: a resting-state functional MR imaging study. Radiology 271(2):543–552
Okada J, Yoshikawa K, Matsuo H, Kanno K, Oouchi M (1991) Reversible MRI and CT findings in uremic encephalopathy. Neuroradiology 33(6):524–526
Pereira AA, Weiner DE, Scott T, Sarnak MJ (2005) Cognitive function in dialysis patients. Am J Kidney Dis 45(3):448–462
Prohovnik I, Post J, Uribarri J, Lee H, Sandu O, Langhoff E (2007) Cerebrovascular effects of hemodialysis in chronic kidney disease. J Cereb Blood Flow Metab 27(11):1861–1869
Qiu Y, Lv X, Su H, Jiang G, Li C, Tian J (2014) Structural and functional brain alterations in end stage renal disease patients on routine hemodialysis: a voxel-based morphometry and resting state functional connectivity study. PLoS One 9(5):e98346
Radić J, Ljutić D, Radić M, Kovaĉić V, Sain M, Curković KD (2010) The possible impact of dialysis modality on cognitive function in chronic dialysis patients. Neth J Med 68(4):153–157
Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001) A default mode of brain function. Proc Natl Acad Sci U S A 98(2):676–682
Sepulcre J, Liu H, Talukdar T, Martincorena I, Yeo BT, Buckner RL (2010) The organization of local and distant functional connectivity in the human brain. PLoS Comput Biol 6(6):e1000808
Snyder AZ, Raichle ME (2012) A brief history of the resting state: the Washington University perspective. NeuroImage 62(2):902–910
Stufflebeam SM, Liu H, Sepulcre J, Tanaka N, Buckner RL, Madsen JR (2011) Localization of focal epileptic discharges using functional connectivity magnetic resonance imaging. J Neurosurg 114(6):1693–1697
Tullberg M, Fletcher E, DeCarli C, et al (2004) White matter lesions impair frontal lobe function regardless of their location. Neurology 63(2):246–253
Wang XJ, Xia MR, Lai YY, et al (2014) Disrupted resting-state functional connectivity in minimally treated chronic schizophrenia. Schizophr Res 156(2–3):150–156
Whitfield-Gabrieli S, Ford JM (2012) Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol 8:49–76
Williams MA, Sklar AH, Burright RG, Donovick PJ (2004) Temporal effects of dialysis on cognitive functioning in patients with ESRD. Am J Kidney Dis 43(4):705–711
Zhang LJ, Wen J, Ni L, et al (2013) Predominant gray matter volume loss in patients with end-stage renal disease: a voxel-based morphometry study. Metab Brain Dis 28(4):647–654
Zheng G, Wen J, Zhang L, et al (2014) Altered brain functional connectivity in hemodialysis patients with end-stage renal disease: a resting-state functional MR imaging study. Metab Brain Dis 29(3):777–786
Zuo XN, Ehmke R, Mennes M, et al (2012) Network centrality in the human functional connectome. Cereb Cortex 22(8):1862–1875
Author contributions
Xiao-Dong Zhang, Ji-Qiu Wen and Qiang Xu contributed equally to this work; Long-Jiang Zhang and Guang-Ming Lu designed the research; Xiao-Dong Zhang, Ji-Qiu Wen, Qiang Xu, Rong-Feng Qi, Hui-Juan Chen, Xiang Kong, Lu-De Wei, Min Xu, Long-Jiang Zhang, Guang-Ming Lu.
Grants
Grants from National Natural Science Foundation of China, Nos. 30700194, 81171313, 81322020 and 81230032 (to Zhang LJ); and Program for New Century Excellent Talents in University, No. NCET-12-0260 (to Zhang LJ).
Conflict of interest
There are no interest conflicts disclosed.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xiao-Dong Zhang, Ji-Qiu Wen, and Qiang Xu had equal contribution to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
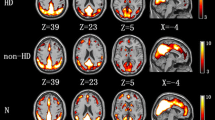
The spatial distribution of long- and short-range FDC in HCs, non-NE and MNE groups. Long-range FCD mainly distributes in bilateral middle/superior frontal gyri, superior parietal gyri, medial prefrontal cortex, anterior cingulate cortex, precuneus/posterior cingulate cortex, postcentral gyrus, inferior parietal lobule, middle temporal gyri, and temporal pole. Short-range FCD mainly locates in bilateral precuneus/posterior cingulate cortex, inferior parietal lobule, middle frontal gyri, Calcarine, middle and superior occipital gyri, medial prefrontal cortex, anterior cingulate cortex, superior parietal gyri, Cuneus, and middle temporal gyri (P < 0.05, AlphaSim corrected). FCD functional connectivity density, HCs healthy control, Non-NE non-nephro-encephalopathy, MNE minimal nephro-encephalopathy. (GIF 91 kb)
Rights and permissions
About this article
Cite this article
Zhang, XD., Wen, JQ., Xu, Q. et al. Altered long- and short-range functional connectivity in the patients with end-stage renal disease: a resting-state functional MRI study. Metab Brain Dis 30, 1175–1186 (2015). https://doi.org/10.1007/s11011-015-9683-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-015-9683-z