Abstract
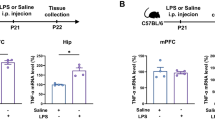
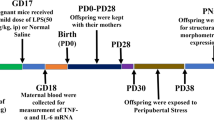
A febrile seizure is a neurological disorder that occurs following an infection that results in a rapid rise in body temperature. It commonly affects 3–5% of children between the ages of 3 months and 5 years. Interleukin-1 beta IL-1β a pro-inflammatory cytokine has been suggested to play a role in the manifestation of febrile seizures. There is evidence suggesting that neurological disorders can be exacerbated in an offspring that was exposed to stress prenatally. The aim of our study was therefore to investigate whether febrile seizures are exacerbated in the offspring of rats that were prenatally stressed. The offspring of pregnant Sprague–Dawley dams were used in the study. Prenatal stress consisted of exposing the pregnant dams to 45 min of restraint, 3 times per day with 3 h intervals in-between, for 7 days starting on gestational day 14 (GND14). On postnatal day (PND) 14, the pups were injected with lipopolysaccharide (LPS, 200 μg/kg, i.p.) followed 2.5 h later by an i.p. injection of kainic acid (KA, 1.75 mg/kg). All the animals were decapitated on PND 21. Trunk blood was collected to detect plasma interleukin-1beta (IL-1β) levels in the various groups. Our data showed that i.p. injections of LPS followed by KA led to the development of seizure activity that was associated with increased plasma IL-1β levels. Prior exposure to prenatal stress resulted in the development of advanced stages of seizure development, leading to an exaggerated seizure response. Prenatal stress alone also led to elevated plasma IL-1β levels, while previously stressed animals receiving LPS and KA yielded the highest plasma levels of IL-1β levels. Our data therefore shows that IL-1β levels may play an important role in the development of febrile seizures.



Similar content being viewed by others
References
Baram TZ, Gerth A, Schultz L (1997) Febrile seizures: an appropriate-aged model suitable for long-term studies. Dev Brain Res 98:265–270
Berger MA, Barros VG, Sarchi MI, Tarazi FI, Antonelli MC (2002) Long-term effects of prenatal stress on dopamine and glutamate receptors in adult rat brain. Neurochem Res 27:1525–1533
Besedovsky HO, Del Rey A, Sorkin E, Dinarello (1986) Immunoregulatory feedback between interleukin-1 and glucocorticoids hormones. Science 233:652–654
Bunch L, Larsen PK (2009) Subtype selective kainic acid receptor agonists: discovery and approaches to rational design. Med Res Rev 29:3–28
Burckingham JC, Loxely HD, Taylor AD, Flower RJ (1994) Cytokines, glucocorticoids and neuroendocrine function. Pharmacol Res 30:35–42
Charila A, Laplante PD, Vaillancourtc C, King S (2010) Prenatal stress and brain development. Brain Res Rev 65:56–79
Diop AG, De Boer HM, Mandlhate C, Prilipko L, Meinardi H (2003) The global campaign against epilepsy in Africa. Acta Trop 87:149–159
Dube C, Vezzani A, Behren M, Bartfai T, Baram TZ (2004) Interleukin-1β contributes to the generation of experimental febrile seizures. Am Neurol Assoc 57:152–155
Dube C, Vezzani A, Behrens M, Bartfai T, Baram TZ (2005) CYTOKINES: a link between fever and seizures. Current literature in basic science. Ann Neurol 57:152–155
Dube CM, Brewster AL, Richichi C, Zha Q, Baram TZ (2007) Fever, febrile seizures and epilepsy. Trends Neurosci 30:491–494
Dube CM, Brewster AL, Baram TZ (2009) Febrile seizures: mechanisms and relationship to epilepsy. Brain Dev 31:366–371
Erakovic V, Zupan G, Varljen J, Laginja J, Simonic A (2001) Altered activities of rat brain metabolic enzymes caused by pentylenetetrazol kindling and pentylenetetrazol — induced seizures. Epilepsy Res 43:165–173
Fumagalli F, Molteni R, Racagni G, Riva MA (2007) Stress during development: impact on neuroplasticity and relevance to psychopathology. Prog Neurobiol 81:197–217
Galic MA, Riazi K, Heida JG, Mouihate A, Fournier NM, Spencer SJ, Kalynchuk LE, Teskey GC, Pittman QJ (2008) Postnatal inflammation increases seizure susceptibility in adult rats. J Neurosci 28(27):6904–6913
Gulec G, Noyan B (2001) Do febrile convulsions decrease the threshold for pilocarpine-induced seizures? Effects of nitric oxide. Dev Brain Res 126:223–228
Heida JG, Pittman QJ (2005) Casual links between brain cytokine and experimental febrile convulsions in the rat. Epilepsia 46(12):1906–1913
Heida JG, Boisse, Pittman JQ (2004) Lipopolysaccharide-induced febrile convulsions in the rat: short term sequelae. Epilepsia 45:1317–1329
Heida JG, Moshe SL, Pittman QJ (2009) The role of interleukin-1β in febrile seizures. Brain Dev 31:388–393
Idro R, Gwer S, Kahindi M, Gatakaa H, Kazungu T, Ndiritu M, Maitland K, Neville BGR, Kager PA, Newton RJC (2008) The incidence, aetiology and outcome of acute seizures in children admitted to a rural Kenyan district hospital. BMC Pediatrics 8:5
Kira R, Torisu H, Takemoto M, Nomura A, Sakai Y, Sanefuji M, Sakamoto K, Matsumoto S, Gondo K, Hara T (2005) Genetic susceptibility to simple febrile seizures: interleukin-1_promotor polymorphisms are associated with sporadic cases. Wiley Interscience 10:1002–20133
Kofman O (2002) The role of prenatal stress in the etiology of developmental behavioural disorders. Neurosci Biobehav Rev 26:457–470
Mabandla MV, Kellaway LA, Daniels WM, Russell VA (2009a) Effect of exercise on dopamineneuron survival in prenatally stressed rats. Metab Brain Dis 24(4):525–39
Mabandla MV, Kellaway LA, Daniels WM, Russell VA (2009b) Effect of exercise on dopamineneuron survival in prenatally stressed rats. Metab Brain Dis 24(4):525–39
Lujan R, Shigemoto, Bendito G (2005) Glutamate and GABA receptor signalling in the developing brain. Neuroscience 130:567–580
Mabandla MV, Russell VA (2010) Voluntary exercise reduces the neurotoxic effects of 6-hydroxydopamine in maternally separatedrats. Behav Brain Res 211:16–22
McEwen BS, Magarinos AM (2001) Stress and hippocampal plasticity: implications for the pathophysiology of affective disorders. Hum Psychopharmacol 16:S7–S19
Ojewole JAO (2008) Anticonvulsant effects of Rhus chirindensis (Baker F.) (Anacardiaceae) stem-bark aqueous extract in mice. J Ethnopharmacol 117:130–135
Patin VA, Lordi B, Caston J (2004a) Does prenatal stress affect the motoric development of the rat pup? Dev Brain Res 149:85–92
Patin VA, Lordi B, Caston J (2004b) Does prenatal stress affect the motoric development of the rat pup? Dev Brain Res 149:85–92
Riazi K, Galic MA, Pittman QJ (2010) Contributions of peripheral inflammation to seizure susceptibility: cytokines and brain excitability. Epilepsy Res 89:34–42
Rijkers K, Majoie HJ, Hoogland G, Kenis G, De Baets M, Vles JS (2009) The role of interleukin-1 in seizures and epilepsy: A critical review. Experimental Neurology 216:258–271
Scantlebury HM, Heida JG (2010) Febrile seizures and temporal lobe epileptogenesis. Epilepsy Res 89:27–33
Spencer SJ, Heida JG, Pittman JQ (2005) Early life challenge-effects on behavioural indices of adult rat fear and axiety. Behav Brain Res 164:231–238
Vezzani A, Baram TZ (2007) New roles for interleukin-1beta in the mechanism of epilepsy. Epilepsy Currents 2:45–50
Vezzani A, Maroso M, Balosso S, Sanchez M, Bartfai T (2011) IL-1receptor/Toll-like receptor signaling in infection, inflammation, stress and neurodegeneration couples hyperexcitability and seizures. Brain Behav Immun 25:1281–1289
Wang J, Dunn AJ (1999) The role of interleukin-6 in the activation of the hypothalamo-pituitary-adrenocortical axis and brain indoleamines by endotoxin and interleukin-1b. Brain Res 815:337–348
Weinstock M (2007) Gender differences in the effects of prenatal stress on brain development and behaviour. Neurochem Res 32:1730–1740
Acknowledgments
The authors wish to thank the Medical Research Council and the National Research Foundation for financial support, as well as the staff of the Biomedical Resource Center of the University of KwaZulu-Natal for technical assistance. This work forms part of Masters degree thesis of one of the authors (L. Qulu).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qulu, L., Daniels, W.M.U. & Mabandla, M.V. Exposure to prenatal stress enhances the development of seizures in young rats. Metab Brain Dis 27, 399–404 (2012). https://doi.org/10.1007/s11011-012-9300-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-012-9300-3