Abstract
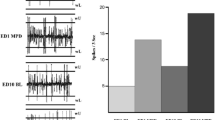
Attention-deficit/hyperactivity disorder (ADHD) is a behavioural disorder that has been associated with dysfunction of the dopaminergic system. Abnormal dopamine function could be the result of a primary defect in dopamine neurons (neuronal firing, dopamine transporter, synthesis, receptor function) or an indirect result of impaired glutamate and/or noradrenergic regulation of dopamine neurons. There is considerable evidence to suggest that dopamine release is impaired at mesolimbic and nigrostriatal dopaminergic terminals. However, it is not known whether dysregulation occurs at the level of the cell bodies in the ventral tegmental area of the midbrain (VTA) and substantia nigra (SN). An in vitro superfusion technique was used to measure dopamine release in a widely used model of ADHD, the spontaneously hypertensive rat (SHR), and its normotensive Wistar-Kyoto (WKY) control. At approximately 30 days of age, rats were analysed for behavioural differences in the open field in response to acute treatment with methylphenidate (0.5 to 2 mg/kg in condensed milk, oral self-administration). In addition, rats were treated chronically with methylphenidate (2 mg/kg, oral self-administration, twice daily for 14 days from postnatal day 21 to 34) before the VTA and the SN were analysed for glutamate—stimulated and depolarization—evoked release of dopamine in these areas. In support of its use as an animal model for ADHD, SHR were more active in the open field and displayed less anxiety-like behaviour than WKY. Neither strain showed any effect of treatment with methylphenidate. A significant difference was observed in glutamate-stimulated release of dopamine in the SN of SHR and WKY, with SHR releasing more dopamine, consistent with the hypothesis of altered glutamate regulation of dopamine neurons in SHR.



Similar content being viewed by others
References
Barr CL, Feng Y, Wigg K, Bloom S, Roberts W, Malone M, Schachar R, Tannock R, Kennedy JL (2000) Identification of DNA variants in the SNAP-25 gene and linkage study of these polymorphisms and attention-deficit hyperactivity disorder. Mol Psychiatry 5:405–409
Bellgrove MA, Hawi Z, Kirley A, Gill M, Robertson IH (2005) Dissecting the attention deficit hyperactivity disorder (ADHD) phenotype: sustained attention, response variability and spatial attentional asymmetries in relation to dopamine transporter (DAT1) genotype. Neuropsychologia 43:1847–1857
Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AFT, Kelley AE, Schmeichel B, Hamilton C, Spencer RC (2006) Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry 60:1111–1120
Brandon CL, Marinelli M, White FJ (2003) Adolescent exposure to methylphenidate alters the activity of rat midbrain dopamine neurons. Biol Psychiatry 54:1338–1344
Carboni E, Silvagni A, Valentini V, Di Chiara G (2003) Effect of amphetamine, cocaine and depolarization by high potassium on extracellular dopamine in the nucleus accumbens shell of SHR rats. An in vivo microdyalisis study. Neurosci Biobehav Rev 27:653–659
Carey MP, Diewald LM, Esposito FJ, Pellicano MP, Carnevale UAG, Sergeant JA, Papa M, Sadile AG (1998) Differential distribution, affinity and plasticity of dopamine D-1 and D-2 receptors in the target sites of the mesolimbic system in an animal model of ADHD. Behav Brain Res 94:173–185
Carter CJ (1982) Topographical distribution of possible glutamatergic pathways from the frontal cortex to the striatum and substantia nigra in rats. Neuropharmacology 21:379–83
Cierpial MA, Shasby DE, Murphy CA, Borom AH, Stewart RE, Swithers SE, McCarty R (1989) Open field behaviour of spontaneously hypertensive and Wistar-Kyoto normotensive rats: effects of reciprocal cross-fostering. Behav Neural Biol 51:203–210
Clements KM, Wainwright PE (2007) Spontaneously hypertensive, Wistar Kyoto and Sprague-Dawley rats differ in performance on a win-stay task and a conditioned cue preference task in the water radial arm maze. Behav Brain Res 183:169–177
Coghill DR, Rhodes SM and Matthews K (2007). The neuropsychological effects of chronic methylphenidate on drug-naïve boys with attention-deficit/ hyperactivity disorder. Biol Psychiatry (Article in Press).
Danysz W, Plaznik A, Pucilowski O, Plewako M, Obersztyn M, Kostowski W (1983) Behavioural studies in spontaneously hypertensive rats. Behav Neural Biol 39:22–29
Dougherty DD, Bonab AA, Spencer TJ, Rauch SL, Madras BK, Fischman AJ (1999) Dopamine transporter density in patients with attention deficit hyperactivity disorder. Lancet 354:2132–2133
Durston S, Tottenham NT, Thomas KM, Davidson MC, Eigsti I-M, Yang Y, Ulug AM, Casey BJ (2003) Differential patterns of striatal activation in young children with and without ADHD. Biol. Psychiatry 53:871–878
Durston S, Hulshoff Pol HE, Schnack HG, Buitelaar JK, Steenhuis MP, Minderaa RB, Kahn RS, van Engeland H (2004) Magnetic resonance imaging of boys with attention deficit/ hyperactivity disorder and their unaffected siblings. J. Am. Acad. Child Adolesc. Psychiatry 43:332–340
Ernst M, Zametkin AJ, Matochik JA, Pascualvaca D, Jons PH, Cohen RM (1999) High midbrain [18F]DOPA accumulation in children with attention deficit hyperactivity disorder. Am J Psychiatry 156:1209–1215
Federici M, Geracitano R, Bernardi G, Mercuri NB (2005) Actions of methylphenidate on dopaminergic neurons of the ventral midbrain. Biol Psychiatry 57:361–365
Ferguson SA, Paule MG, Cada A, Fogle CM, Gray EP and Berry KJ (2007). Baseline behaviour, but not sensitivity to stimulant drugs, differs among Spontaneously Hypertensive, Wystar Kyoto and Sprague Dawley rat strains. Neurotoxicol Teratol (Article in Press).
Gerasimov MR, Franceschi M, Volkow ND, Gifford A, Gatley SJ, Marsteller D, Molina PE, Dewey SL (2000) Comparison between intraperitoneal and oral methylphenidate administration: a microdialysis and locomotor activity study. In J Pharmacol Exp Ther 295:51–57
Hatzipetros T, Yamamoto BK (2006) Dopaminergic and GABAergic modulation of glutamate release from rat subthalamic nucleus efferents to the substantia nigra. Brain Res. 1076:60–7
Heise CE, Mitrofanis J (2004) Evidence for a glutamatergic projection from the zona incerta to the basal ganglia of rats. J Comp Neurol. 468(4):482–95
Hood J, Baird G, Rankin PM, Isaacs E (2005) Immediate effects of methylphenidate on cognitive attention skills of children with attention deficit hyperactivity disorder. Dev Med Child Neurol 47:408–414
Johansen EB, Aase H, Meyer A, Sagvolden T (2002) Attention-deficit/ hyperactivity disorder (ADHD) behaviour explained by dysfunctioning reinforcement and extinction processes. Behav Brain Res 130:37–45
Kieling C, Roman T, Doyle AE, Hutz MH, Rohde LA (2006) Association between DRD4 gene and performance of children with ADHD in a test of sustained attention. Biol Psychiatry 60:1163–1165
Knardahl S, Sagvolden T (1979) Open-field behaviour of spontaneously hypertensive rats. Behav Neural Biol 27:187–200
Krause K-H, Dresel SH, Krause J, Kung HF, Tatsch K (2000) Increased striatal dopamine transporter in adult patients with attention-deficit hyperactivity disorder: effects of methylphenidate as measured by single photon emission computed tomography. Neurosci Lett 285:107–110
Kuczenski R, Segal DS (2005) Stimulant actions in rodents: implications for attention deficit hyperactivity disorder treatment and potential substance abuse. Biol Psychiatry 57:1391–1396
Lasky-Su J, Neale BM, Franke B, Anney RJ, Zhou K, Maller JB et al (2008) Genome-wide association scan of quantitative traits for attention deficit hyperactivity disorder identifies novel associations and confirms candidate gene associations. Am J Med Genet B Neuropsychiatr Genet 147B:1345–1354
Leo D, Sorrentino E, Volpicelli F, Eyman M, Greco D, Viggiano D, di Porzio U, Perrone-Capano C (2003) Altered midbrain dopaminergic neurotransmission during development in an animal model of ADHD. Neurosci Biobehav Rev 27:661–669
Li Q, Lu G, Antonio GE, Mak YT, Rudd JA, Fan M, Yew DT (2007) The usefulness of the spontaneously hypertensive rat to model attention-deficit/hyperactivity disorder (ADHD) may be explained by the differential expression of dopamine-related genes in the brain. Neurochem Int 50:848–857
Lijffijt M, Kenemans JL, ter Wal A, Quik EH, Kemner C, Westenberg H, Verbaten MN, van Engeland H (2006) Dose-related effect of methylphenidate on stopping and changing in children with attention-deficit/ hyperactivity disorder. Eur Psychiatry 21:544–547
Lindvall O, Björklund A (1978) Anatomy of the dopaminergic neuron systems in the rat brain. Adv Biochem Psychopharmacol. 19:1–23
Mazei-Robison MS, Couch RS, Shelton RC, Stein MA, Blakely RD (2005) Sequence variation in the human dopamine transporter gene in children with attention deficit hyperactivity disorder. Neuropharmacology 49:724–736
Ohadi M, Shirazi E, Tehranodoosti M, Moghimi N, Keikhaee MR, Ehssani S, Aghajani A, Najmabadi H (2006) Attention-deficit/ hyperactivity disorder (ADHD) association with the DAT1 core promoter -67 T allele. Brain Res 1101:1–4
Okamoto K, Aoki K (1963) Development of a strain of spontaneously hypertensive rats. Jpn Circ J 27:282–293
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates. Academic, San Diego
Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA (2007) The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry 164:942–948
Ramos A, Morméde P (1998) Stress and emotionality: a multidimensional and genetic approach. Neurosci Biobehav Rev 22:33–57
Ramos A, Berton O, Mormede P, Chaouloff F (1997) A multiple-test study of anxiety-related behaviours in six inbred rat strains. Behav Brain Res 85:57–69
Rosa-Neto P, Lou HC, Cumming P, Pryds O, Karrebaek H, Lunding J, Gjedde A (2005) Methylphenidate-evoked changes in striatal dopamine correlate with inattention and impulsivity in adolescents with attention-deficit hyperactivity disorder. Neuroimage 25:868–876
Ruskin DN, Bergstrom DA, Shenker A, Freeman LE, Baek D, Walters JR (2001) Drugs used in the treatment of attention-deficit/ hyperactivity disorder affect postsynaptic firing rate and oscillation without preferential dopamine autoreceptor action. Biol Psychiatry 49:340–350
Russell VA (2000) The nucleus accumbens motor-limbic interface of the spontaneously hypertensive rat as studied in vitro by the superfusion slice technique. Neurosci Biobehav Rev 24:133–136
Russell VA (2003) In vitro glutamate-stimulated release of dopamine from nucleus accumbens core and shell of spontaneously hypertensive rats. Metab Brain Dis. 18:161–168
Russell VA (2007) Neurobiology of animal models of attention-deficit hyperactivity disorder. J Neurosci Methods 161:185–198
Russell V, de Villiers A, Sagvolden T, Lamm M, Taljaard J (1995) Altered dopaminergic function in the prefrontal cortex, nucleus accumbens and caudate-putamen of an animal model of attention-deficit hyperactivity disorder—the spontaneously hypertensive rat. Brain Res 676:343–451
Russell V, de Villiers A, Sagvolden T, Lamm M, Taljaard J (1998) Differences between electrically-, Ritalin- and D-amphetamine-stimulated release of [3H]-dopamine from brain slices suggests impaired vesicular storage of dopamine in an animal model of attention-deficit hyperactivity disorder. Behav Brain Res 94:163–171
Russell VA, de Villiers AS, Sagvolden T, Lamm MCL, Taljaard JJF (2000) Methylphenidate affects striatal dopamine differently in an animal model for attention-deficit/ hyperactivity disorder—the spontaneously hypertensive rat. Brain Res Bull 53:187–192
Russell VA, Sagvolden T, Johansen EB (2005) Animal models of attention-deficit hyperactivity disorder. Behav Brain Function 1:9
Sagvolden T (2000) Behavioural validation of the spontaneously hypertensive rat (SHR) as an animal model of attention deficit/ hyperactivity disorder (AD/HD). Neurosci Biobehav Rev 24:31–39
Sagvolden T, Sergeant JA (1998) Attention deficit hyperactivity disorder—from brain dysfunction to behaviour. Behav Brain Res 94:1–10
Sagvolden T, Johansen EB, Aase H, Russell VA (2005a) A dynamic developmental theory of Attention-Deficit/Hyperactivity Disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behav. Brain Sci. 28:397–419
Sagvolden T, Russell VA, Aase A, Johansen EB, Farshbaf M (2005b) Rodent models of attention-deficit/hyperactivity disorder. Biol. Psychiatry 57:1239–1247
Scarnati E, Proia A, Campana E, Pacitti C (1986) A microiontophoretic study on the nature of the putative synaptic neurotransmitter involved in the pedunculopontine-substantia nigra pars compacta excitatory pathway of the rat. Exp. Brain Res. 62:470–8
Shen R-Y, Choong K-C (2006) Different adaptations in ventral tegmental area dopamine neurons in control and ethanol exposed rats after methylphenidate treatment. Biol Psychiatry 59:635–642
Solanto MV (1998) Neuropsychopharmacological mechanisms of stimulant drug action in attention-deficit hyperactivity disorder: a review and integration. Behav Brain Res 94:127–152
Spencer TJ, Biederman J, Madras BK, Dougherty DD, Bonab AA, Livni E, Meltzer PC, Martin J, Rauch S, Fischman AJ (2007) Further evidence of dopamine transporter dysregulation in ADHD: a controlled PET imaging study using altropane. Biol. Psychiatry 62:1059–1061
Sunohara GA, Malone MA, Rover J, Humphries T, Roberts W, Taylor MJ (1999) Effect of methylphenidate on attention in children with attention deficit hyperactivity disorder (ADHD): ERP evidence. Neuropsychopharmacology 21:218–228
Swanson J, Castellanos FX, Murias M, LaHoste G, Kennedy J (1998) Cognitive neuroscience of attention deficit hyperactivity disorder and hyperkinetic disorder. Curr Opin Neurobiol 8:263–271
Swanson JM, Kinsbourne M, Nigg J, Lanphear B, Stefanatos GA, Volkow N, Taylor E, Casey BJ, Castellanos FX, Wadhwa PD (2007) Etiologic subtypes of attention-deficit/hyperactivity disorder: brain imaging, molecular genetic and environmental factors and the dopamine hypothesis. Neuropsychol Rev 17:39–59
Thapar A, O’Donovan M, Owen MJ (2005) The genetics of attention deficit hyperactivity disorder. Hum Mol Genet 14(2):275–282
Tsai S-J (2006) Signal transducer and activator of transcription 6 (STAT6) and attention-deficit hyperactivity disorder: a speculative hypothesis. Med Hypotheses 67:1341–1343
Van der Kooij MA, Glennon JC (2007) Animal models concerning the role of dopamine in attention-deficit hyperactivity disorder. Neurosci Biobehav Rev 31:597–618
Van‘t Ent D, Lehn H, Derks EM, Hudziak JJ, van Strien NM, Veltman DJ, De Geus EJC, Todd RD, Boomsma DI (2007) A structural MRI study in monozygotic twins concordant or discordant for attention/ hyperactivity problems: evidence for genetic and environmental heterogeneity in the developing brain. Neuroimage 35:1004–1020
Volkow ND, Wang G-J, Newcorn J, Fowler JS, Telang F, Solanto MV, Logan J, Wong C, Ma Y, Swanson JM, Schultz K, Pradhan K (2007) Brain dopamine transporter levels in treatment and drug naïve adults with ADHD. Neuroimage 34:1182–1190
Watanabe Y, Fujita M, Ito Y, Okada T, Kusuoka H, Nishimura T (1997) Brain dopamine transporter in spontaneously hypertensive rats. J Nucl Med 38:470–474
Wilens TE, Biederman J, Spencer TJ (2002) Attention deficit/ hyperactivity disorder across the lifespan. Annu Rev Med 53:113–131
Wise R (2008) Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox. Res. 14:169–83
Wultz B, Sagvolden T, Moser E, Moser M-B (1990) The spontaneously hypertensive rat as an animal model of attention-deficit hyperactivity disorder: effects of methylphenidate on exploratory behaviour. Behav Neural Biol 53:88–102
Yang PB, Amini B, Swann AC, Dafny N (2003) Strain differences in the behavioural responses of male rats to chronically administered methylphenidate. Brain Res 971:139–152
Yang J-W, Jang W-S, Hong SD, Ji YI, Kim DH, Park J, Kim SW, Joung YS (2008) A case-control association study of the polymorphism at the promoter region of the DRD4 gene in Korean boys with attention deficit hyperactivity disorder: evidence of association with the -521 C/T SNP. Prog Neuropsychopharmacol Biol Psychiatry 32:243–248
Acknowledgments
This work was supported by the South African Medical Research Council and the University of Cape Town. The authors would also like to thank Mr Letlogonolo Selaledi and Mrs Nuraan Ismail for assistance with animal care. Miss Fleur Warton was the recipient of a National Research Foundation scholarship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Warton, F.L., Howells, F.M. & Russell, V.A. Increased glutamate-stimulated release of dopamine in substantia nigra of a rat model for attention-deficit/hyperactivity disorder—lack of effect of methylphenidate. Metab Brain Dis 24, 599–613 (2009). https://doi.org/10.1007/s11011-009-9166-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-009-9166-1