Abstract
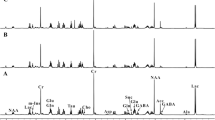
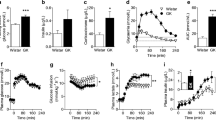
Glucose loading in thiamine-deficient patients is known to precipitate Wernicke’s Encephalopathy; however, the mechanisms responsible have not been fully elucidated. Lactate accumulation occurs in brains of thiamine-deficient rats. In order to determine whether glucose loading in thiamine-deficient rats causes selective lactic acidosis in vulnerable brain structures, cerebral pH was measured autoradiographically using 14-labeled 5,5-dimethyloxazolidine-2, 4-dione ([14C]DMO) in the medial thalamus, a vulnerable brain region, versus cerebral cortex, a brain region that is spared in thiamine deficiency. Following administration of a glucose load, regional lactate levels and de novo lactate synthesis measured by 1H-13C-NMR spectroscopy, increased significantly to 21.86 ± 3.04 μmol/g (wet weight) in the medial thalamus (p < 0.001) and pH in this brain region was decreased significantly from 7.08 ± 0.04 to 6.87 ± 0.05 (p < 0.001). No such changes were observed in cerebral cortex following a glucose load. These results demonstrate that the increased production and accumulation of brain lactate result in acidosis following glucose loading in thiamine deficiency. Alterations of brain pH could contribute to the pathogenesis of thalamic neuronal damage and consequent cerebral dysfunction in Wernicke’s Encephalopathy.

Similar content being viewed by others
References
Butterworth RF (1989) Effects of thiamine deficiency on brain metabolism: implications for the pathogenesis of the Wernicke-Korsakoff syndrome. Alcohol Alcohol 24:271–279
Butterworth RF, Heroux M (1989) Effect of pyrithiamine treatment and subsequent thiamine rehabilitation on regional cerebral amino acids and thiamine-dependent enzymes. J Neurochem 52:1079–1084
Butterworth RF, Giguere JF, Besnard AM (1985) Activities of thiamine-dependent enzymes in two experimental models of thiamine-deficiency encephalopathy: 1. The pyruvate dehydrogenase complex. Neurochem Res 10:1417–1428
Butterworth RF, Giguere JF, Besnard AM (1986) Activities of thiamine-dependent enzymes in two experimental models of thiamine-deficiency encephalopathy. 2. alpha-Ketoglutarate dehydrogenase. Neurochem Res 11:567–577
Butterworth RF, Gaudreau C, Vincelette J, Bourgault AM, Lamothe F, Nutini AM (1991) Thiamine deficiency and Wernicke’s encephalopathy in AIDS. Metab Brain Dis 6:207–212
Calingasan NY, Huang PL, Chun HS, Fabian A, Gibson GE (2000) Vascular factors are critical in selective neuronal loss in an animal model of impaired oxidative metabolism. J Neuropathol Exp Neurol 59:207–217
Gibson GE, Ksiezak-Reding H, Sheu KF, Mykytyn V, Blass JP (1984) Correlation of enzymatic, metabolic, and behavioral deficits in thiamin deficiency and its reversal. Neurochem Res 9:803–814
Hakim AM (1984) The induction and reversibility of cerebral acidosis in thiamine deficiency. Ann Neurol 16:673–679
Holowach J, Kauffman F, Ikossi MG, Thomas C, McDougal DB Jr (1968) The effects of a thiamine antagonist, pyrithiamine, on levels of selected metabolic intermediates and on activities of thiamine-dependent enzymes in brain and liver. J Neurochem 15:621–631
Katzman R, Pappius HM (1973) Brain Electrolytes and Fluid Metabolism. Williams & Wilkins, Baltimore
Kobatake K, Sako K, Izawa M, Yamamoto YL, Hakim AM (1984) Autoradiographic determination of brain pH following middle cerebral artery occlusion in the rat. Stroke 15:540–547
Kogure K, Alonso OF, Martinez E (1980) A topographic measurement of brain pH. Brain Res 195:95–109
Kontos HA (1985) George E. Brown memorial lecture. Oxygen radicals in cerebral vascular injury. Circ Res 57:508–516
Kontos HA (1989) Oxygen radicals in CNS damage. Chem Biol Interact 72:229–255
Kruse M, Navarro D, Desjardins P, Butterworth RF (2004) Increased brain endothelial nitric oxide synthase expression in thiamine deficiency: relationship to selective vulnerability. Neurochem Int 45:49–56
Kuschinsky W, Wahl M, Bosse O, Thurau K (1972) Perivascular potassium and pH as determinants of local pial arterial diameter in cats. A microapplication study. Circ Res 31:240–247
Luxemburger C, White NJ, ter Kuile F, Singh HM, Allier-Frachon I, Ohn M, Chongsuphajaisiddhi T, Nosten F (2003) Beri-beri: the major cause of infant mortality in Karen refugees. Trans R Soc Trop Med Hyg 97:251–255
McCandless DW (1982) Energy metabolism in the lateral vestibular nucleus in pyrithiamin-induced thiamin deficiency. Ann N Y Acad Sci 378:355–364
McCandless DW, Schenker S, Cook M (1968) Encephalopathy of thiamine deficiency: studies of intracerebral mechanisms. J Clin Invest 47:2268–2280
McGready R, Simpson JA, Cho T, Dubowitz L, Changbumrung S, Bohm V, Munger RG, Sauberlich HE, White NJ, Nosten F (2001) Postpartum thiamine deficiency in a Karen displaced population. Am J Clin Nutr 74:808–813
Munujos P, Coll-Canti J, Beleta J, Gonzalez-Sastre F, Gella FJ (1996) Brain pyruvate oxidation in experimental thiamin-deficiency encephalopathy. Clin Chim Acta 255:13–25
Myers RE (1979) A unitary theory of causation of anoxic and hypoxic brain pathology. Adv Neurol 26:195–213
Navarro D, Zwingmann C, Hazell AS, Butterworth RF (2005) Brain lactate synthesis in thiamine deficiency: a re-evaluation using 1H-13C nuclear magnetic resonance spectroscopy. J Neurosci Res 79:33–41
Rehncrona S, Rosen I, Siesjo BK (1980) Excessive cellular acidosis: an important mechanism of neuronal damage in the brain? Acta Physiol Scand 110:435–437
Rehncrona S, Rosen I, Siesjo BK (1981) Brain lactic acidosis and ischemic cell damage: 1. Biochemistry and neurophysiology. J Cereb Blood Flow Metab 1:297–311
Roos A (1971) Intracellular pH and buffering power of rat brain. Am J Physiol 221:176–181
Schroth G, Wichmann W, Valavanis A (1991) Blood-brain-barrier disruption in acute Wernicke encephalopathy: MR findings. J Comput Assist Tomogr 15:1059–1061
Todd K, Butterworth RF (1999) Mechanisms of selective neuronal cell death due to thiamine deficiency. Ann N Y Acad Sci 893:404–411
Troncoso JC, Johnston MV, Hess KM, Griffin JW, Price DL (1981) Model of Wernicke’s encephalopathy. Arch Neurol 38:350–354
Van Nimmen D, Weyne J, Demeester G, Leusen I (1986) Local cerebral glucose utilization during intracerebral pH changes. J Cereb Blood Flow Metab 6:584–589
Wallis WE, Willoughby E, Baker P (1978) Coma in the Wernicke-Korsakoff syndrome. Lancet 2:400–401
Watson AJ, Walker JF, Tomkin GH, Finn MM, Keogh JA (1981) Acute Wernickes encephalopathy precipitated by glucose loading. Ir J Med Sci 150:301–303
Zelaya FO, Rose SE, Nixon PF, Wholohan BT, Bower AJ, Zimitat C, Schoutrop J, Doddrell DM (1995) MRI demonstration of impairment of the blood-CSF barrier by glucose administration to the thiamin-deficient rat brain. Magn Reson Imaging 13:555–561
Acknowledgements
Research funded by the Canadian Institutes for Health Research (CIHR). We thank Professor Dieter Leibfritz, University of Bremen, for the generous availability of the NMR laboratory.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Navarro, D., Zwingmann, C., Chatauret, N. et al. Glucose loading precipitates focal lactic acidosis in the vulnerable medial thalamus of thiamine-deficient rats. Metab Brain Dis 23, 115–122 (2008). https://doi.org/10.1007/s11011-007-9076-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-007-9076-z