Abstract
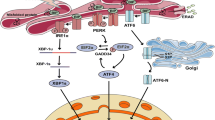
Brefeldin A (BFA) disrupts the structure of the Golgi apparatus to trigger ER stress signaling pathways. On the other hand, treatment with BFA induces the activation of CREB3, the protein structure of which is similar to that of ATF6. In this study, we established Neuro2a cells in which three different transcription factors, namely, ATF4, ATF3 and CREB3, were deficient using the CRISPR/Cas9 approach, and we investigated the BFA-induced ER and Golgi stress response in these cells. BFA treatment rapidly induced ATF4, ATF3, Herp and GADD153 protein expression in Neuro2a cells. ATF4-deficient Neuro2a cells exhibited significantly decreased mRNA and protein expression of ATF3 and Herp but not GADD153; however, cells deficient in ATF3 exhibited minimal effects on GADD34, GADD153 and Herp expression. The cleavage of CREB3 in Neuro2a cells was triggered by BFA; however, the expression of several ER and Golgi stress-related factors was hardly influenced by the CREB3 deficiency in these Neuro2a cells. This study shows that CREB3 minimally associates with typical ER stress-inducible responses in Neuro2a cells. Therefore, identification and characterization of the downstream transcriptional targets of CREB3 is required to clarify not only Golgi stress response but also its relationship with ER stress signaling pathways.







Similar content being viewed by others
Data availability
The data generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ATF3:
-
Activating transcription factor 3
- ATF4:
-
Activating transcription factor 4
- ATF6:
-
Activating transcription factor 6
- CREB3:
-
cAMP response element binding protein 3
- CRISPR/Cas9:
-
Clustered regularly interspaced short palindromic repeats/CRISPR-associated proteins 9
- ER:
-
Endoplasmic reticulum
- ERAD:
-
ER stress-associated degradation
- ERSE:
-
ER stress response element
- GADD153:
-
Growth arrest and DNA damage inducible gene 153
- GRP78:
-
78 KDa glucose-regulated protein
- G3PDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- Herp:
-
Homocysteine-induced ER protein
- IRE1:
-
Inositol-requiring enzyme-1
- PERK:
-
PKR-like endoplasmic reticulum kinase
- TFE3:
-
Transcription factor E3
- RT-PCR:
-
Reverse transcription polymerase chain reaction
- XBP1:
-
X-box binding protein 1
References
Helenius A, Marquardt T, Braakman I (1992) The endoplasmic reticulum as protein-folding compartment. Trends Cell Biol 2:227–231
Gething MJ, Sambrook J (1992) Protein folding in the cell. Nature 355:33–45
Harding HP, Zhang Y, Ron D (1999) Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397:271–274
Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K (2001) XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107:881–891
Zhu C, Johansen FE, Prywes R (1997) Interaction of ATF6 and serum response factor. Mol Cell Biol 17:4957–4966
Lee AH, Iwakoshi NN, Glimcher LH (2003) XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol 23:7448–7459
Okada T, Yoshida H, Akazawa R, Negishi M, Mori K (2002) Distinct roles of activating transcription factor 6 (ATF6) and double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase (PERK) in transcription during the mammalian unfolded protein response. Biochem J 366:585–594
Lange PS, Chavez JC, Pinto JT, Coppola G, Sun CW, Townes TM, Geschwind DH, Ratan RR (2008) ATF4 is an oxidative stress-inducible, prodeath transcription factor in neurons in vitro and in vivo. J Exp Med 205:1227–1242
Taniguchi M, Yoshida H (2017) TFE3, HSP47, and CREB3 pathways of the mammalian Golgi stress response. Cell Struct Funct 42:27–36
Reiling JH, Olive AJ, Sanyal S, Carette JE, Brummelkamp TR, Ploegh HL, Starnbach MN, Sabatini DM (2013) A CREB3-ARF4 signalling pathway mediates the response to Golgi stress and susceptibility to pathogens. Nat Cell Biol 15:1473–1485
Helms JB, Rothman JE (1992) Inhibition by brefeldin A of a Golgi membrane enzyme that catalyses exchange of guanine nucleotide bound to ARF. Nature 360:352–354
Oh-hashi K, Sugiura N, Amaya F, Isobe KI, Hirata Y (2018) Functional validation of ATF4 and GADD34 in Neuro2a cells by CRISPR/Cas9-mediated genome editing. Mol Cell Biochem 440:65–75
Duksin D, Bornstein P (1977) Impaired conversion of procollagen to collagen by fibroblasts and bone treated with tunicamycin, an inhibitor of protein glycosylation. J Biol Chem 252:955–962
Vattem KM, Wek RC (2004) Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci USA 101:11269–11274
Jiang HY, Wek SA, McGrath BC, Lu D, Hai T, Harding HP, Wang X, Ron D, Cavener DR, Wek RC (2004) Activating transcription factor 3 is integral to the eukaryotic initiation factor 2 kinase stress response. Mol Cell Biol 24:1365–1377
Lu R, Yang P, O’Hare P, Misra V (1997) Luman, a new member of the CREB/ATF family, binds to herpes simplex virus VP16-associated host cellular factor. Mol Cell Biol 17:5117–5126
Oh-hashi K, Soga A, Naruse Y, Takahashi K, Kiuchi K, Hirata Y (2018) Elucidating post-translational regulation of mouse CREB3 in Neuro2a cells. Mol Cell Biochem 448:287–297
Kokame K, Agarwala KL, Kato H, Miyata T (2000) Herp, a new ubiquitin-like membrane protein induced by endoplasmic reticulum stress. J Biol Chem 275:32846–32853
Ma Y, Hendershot LM (2004) Herp is dually regulated by both the endoplasmic reticulum stress-specific branch of the unfolded protein response and a branch that is shared with other cellular stress pathways. J Biol Chem 279:13792–13799
Chan SL, Fu W, Zhang P, Cheng A, Lee J, Kokame K, Mattson MP (2004) Herp stabilizes neuronal Ca2+ homeostasis and mitochondrial function during endoplasmic reticulum stress. J Biol Chem 279:28733–28743
Liang G, Audas TE, Li Y, Cockram GP, Dean JD, Martyn AC, Kokame K, Lu R (2006) Luman/CREB3 induces transcription of the endoplasmic reticulum (ER) stress response protein Herp through an ER stress response element. Mol Cell Biol 26:7999–8010
Hoseki J, Ushioda R, Nagata K (2010) Mechanism and components of endoplasmic reticulum associated degradation. J Biochem 147:19–25
Esvelt KM, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Church G (2013) RNA-guided human genome engineering via Cas9. Science 339:823–826
Luethy JD, Fargnoli J, Park JS, Fornace AJ Jr, Holbrook NJ (1990) Isolation and characterization of the hamster gadd153 gene. Activation of promoter activity by agents that damage DNA. J Biol Chem 265(27):16521–16526
Ma Y, Brewer JW, Diehl JA, Hendershot LM (2002) Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J Mol Biol 318:1351–1365
Guyton KZ, Xu Q, Holbrook NJ (1996) Induction of the mammalian stress response gene GADD153 by oxidative stress: role of AP-1 element. Biochem J 314:547–554
Liu G, Su L, Hao X, Zhong N, Zhong D, Singhal S, Liu X (2012) Salermide up-regulates death receptor 5 expression through the ATF4-ATF3-CHOP axis and leads to apoptosis in human cancer cells. J Cell Mol Med 16:1618–1628
Tian F, Zhao J, Bu S, Teng H, Yang J, Zhang X, Li X, Dong L (2020) KLF6 induces apoptosis in human lens epithelial cells through the ATF4-ATF3-CHOP axis. Drug Des Dev Ther 14:1041–1055
Haze K, Yoshida H, Yanagi H, Yura T, Mori K (1999) Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Cell Biol 10:3787–3799
Lu R, Misra V (2000) Potential role for luman, the cellular homologue of herpes simplex virus VP16 (alpha gene trans-inducing factor), in herpesvirus latency. J Virol 74:934–943
Asada R, Kanemoto S, Kondo S, Saito A, Imaizumi K (2011) The signalling from endoplasmic reticulum-resident bZIP transcription factors involved in diverse cellular physiology. J Biochem 149:507–518
Oh-hashi K, Takahashi K, Hirata Y (2019) Regulation of the ER-bound transcription factor Luman/CREB3 in HEK293 cells. FEBS Lett 593:2771–2778
Taniguchi M, Nadanaka S, Tanakura S, Sawaguchi S, Midori S, Kawai Y, Yamaguchi S, Shimada Y, Nakamura Y, Matsumura Y, Fujita N, Araki N, Yamamoto M, Oku M, Wakabayashi S, Kitagawa H, Yoshida H (2015) TFE3 is a bHLH-ZIP-type transcription factor that regulates the mammalian Golgi stress response. Cell Struct Funct 40:13–30
DenBoer LM, Hardy-Smith PW, Hogan MR, Cockram GP, Audas TE, Lu R (2005) Luman is capable of binding and activating transcription from the unfolded protein response element. Biochem Biophys Res Commun 331:113–119
Yamamoto K, Yoshida H, Kokame K, Kaufman RJ, Mori K (2004) Differential contributions of ATF6 and XBP1 to the activation of endoplasmic reticulum stress-responsive cis-acting elements ERSE, UPRE and ERSE-II. J Biochem 136:343–350
Martina JA, Diab HI, Brady OA, Puertollano R (2016) TFEB and TFE3 are novel components of the integrated stress response. EMBO J 35:479–495
Hu Y, Chu L, Liu J, Yu L, Song SB, Yang H, Han F (2019) Knockdown of CREB3 activates endoplasmic reticulum stress and induces apoptosis in glioblastoma. Aging (Albany NY) 11:8156–8168
Ying Z, Zhai R, McLean NA, Johnston JM, Misra V, Verge VM (2015) The unfolded protein response and cholesterol biosynthesis link Luman/CREB3 to regenerative axon growth in sensory neurons. J Neurosci 35:14557–14570
Wu Y, Xie Z, Chen J, Chen J, Ni W, Ma Y, Huang K, Wang G, Wang J, Ma J, Shen S, Fan S (2019) Circular RNA circTADA2A promotes osteosarcoma progression and metastasis by sponging miR-203a-3p and regulating CREB3 expression. Mol Cancer 18:73
Acknowledgements
This work is, in part, supported by Grant-in-aid from the Japan Society for the Promotion of Science (JSPS, Japan, KAKENHI, No. 19H04030 to K.O.).
Author information
Authors and Affiliations
Contributions
KO, TH and KT discussed and designed the research; KO, TH, YM and KT performed experiments; KO and YH confirmed the results; KO and TH prepared the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Oh-hashi, K., Hasegawa, T., Mizutani, Y. et al. Elucidation of brefeldin A-induced ER and Golgi stress responses in Neuro2a cells. Mol Cell Biochem 476, 3869–3877 (2021). https://doi.org/10.1007/s11010-021-04187-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-021-04187-1