Abstract
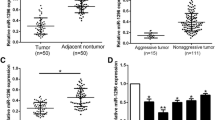
The study aimed to explore the relationship between miR-9-5p and ESR1, and clarify the underlying functional mechanism in the occurrence and development of hepatocellular carcinoma (HCC). Expression data including miRNAs and mRNAs of HCC downloaded from TCGA database were processed for differential analysis, and corresponding clinical data were collected for survival analysis to identify the target miRNA miR-9-5p. Bioinformatics databases were applied for predicting downstream target mRNAs of miR-9-5p. qRT-PCR was used to evaluate expression of miR-9-5p. Western blot was used to detect protein expression of ESR1. MTT, wound healing assay and Transwell assay were used to detect HCC cell proliferation, migration and invasion, respectively. Dual-luciferase reporter gene assay was used to identify the targeting relationship between miR-9-5p and ESR1. Research suggested that miR-9-5p was highly expressed in HCC cells but ESR1 was poorly expressed. Overexpression of miR-9-5p could improve the proliferation, invasion and migration of cells. Dual-luciferase reporter assay showed that ESR1 was the downstream target of miR-9-5p in HCC. Overexpression of miR-9-5p markedly reduced ESR1 mRNA and protein levels in HCC cells, whereas inhibition of miR-9-5p expression produced the contrary results. Silencing ESR1 could noticeably reverse the effect of miR-9-5p knockdown on the proliferation, migration and invasion of HCC cells. As an oncogene, miR-9-5p fostered the proliferation, migration and invasion of HCC cells by targeting and inhibiting ESR1 expression.




Similar content being viewed by others
Data availability
The data and materials in the current study are available from the corresponding author on reasonable request.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Cheng Z, Li X, Ding J (2016) Characteristics of liver cancer stem cells and clinical correlations. Cancer Lett 379:230–238
Fu J, Wang H (2018) Precision diagnosis and treatment of liver cancer in China. Cancer Lett 412:283–288
Nourmohammadi B, Tafsiri E, Rahimi A, Nourmohammadi Z, Daneshvar Kakhaki A, Cho W, Karimipoor M (2019) Expression of miR-9 and miR-200c, ZEB1, ZEB2 and E-cadherin in Non-Small Cell Lung Cancers in Iran. Asian Pac J Cancer Prev 20:1633–1639
Khafaei M, Rezaie E, Mohammadi A, Shahnazi Gerdehsang P, Ghavidel S, Kadkhoda S, Zorrieh Zahra A, Forouzanfar N, Arabameri H, Tavallaie M (2019) miR-9: from function to therapeutic potential in cancer. J Cell Physiol 234:14651–14665
Chen L, Hu W, Li G, Guo Y, Wan Z, Yu J (2019) Inhibition of miR-9-5p suppresses prostate cancer progress by targeting StarD13. Cell Mol Biol Lett 24:20
Azizmohammadi S, Safari A, Azizmohammadi S, Kaghazian M, Sadrkhanlo M, Yahaghi E, Farshgar R, Seifoleslami M (2017) Molecular identification of miR-145 and miR-9 expression level as prognostic biomarkers for early-stage cervical cancer detection. QJM 110:11–15
Barbano R, Pasculli B, Rendina M, Fontana A, Fusilli C, Copetti M, Castellana S, Valori VM, Morritti M, Graziano P, Luigi C, Coco M, Picardo F, Mazza T, Evron E, Murgo R, Maiello E, Esteller M, Fazio VM, Parrella P (2017) Stepwise analysis of MIR9 loci identifies miR-9-5p to be involved in oestrogen regulated pathways in breast cancer patients. Sci Rep 7:45283
Xie CH, Cao YM, Huang Y, Shi QW, Guo JH, Fan ZW, Li JG, Chen BW, Wu BY (2016) Long non-coding RNA TUG1 contributes to tumorigenesis of human osteosarcoma by sponging miR-9-5p and regulating POU2F1 expression. Tumour Biol 37:15031–15041
Sun Z, Han Q, Zhou N, Wang S, Lu S, Bai C, Zhao RC (2013) MicroRNA-9 enhances migration and invasion through KLF17 in hepatocellular carcinoma. Mol Oncol 7:884–894
Han D, Li J, Wang H, Su X, Hou J, Gu Y, Qian C, Lin Y, Liu X, Huang M, Li N, Zhou W, Yu Y, Cao X (2017) Circular RNA circMTO1 acts as the sponge of microRNA-9 to suppress hepatocellular carcinoma progression. Hepatology 66:1151–1164
Ma L, Young J, Prabhala H, Pan E, Mestdagh P, Muth D, Teruya-Feldstein J, Reinhardt F, Onder TT, Valastyan S, Westermann F, Speleman F, Vandesompele J, Weinberg RA (2010) miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol 12:247–256
Zhou B, Xu H, Xia M, Sun C, Li N, Guo E, Guo L, Shan W, Lu H, Wu Y, Li Y, Yang D, Weng D, Meng L, Hu J, Ma D, Chen G, Li K (2017) Overexpressed miR-9 promotes tumor metastasis via targeting E-cadherin in serous ovarian cancer. Front Med 11:214–222
Li G, Wu F, Yang H, Deng X, Yuan Y (2017) MiR-9-5p promotes cell growth and metastasis in non-small cell lung cancer through the repression of TGFBR2. Biomed Pharmacother 96:1170–1178
Wang J, Wang B, Ren H, Chen W (2019) miR-9-5p inhibits pancreatic cancer cell proliferation, invasion and glutamine metabolism by targeting GOT1. Biochem Biophys Res Commun 509:241–248
Jeselsohn R, Buchwalter G, De Angelis C, Brown M, Schiff R (2015) ESR1 mutations-a mechanism for acquired endocrine resistance in breast cancer. Nat Rev Clin Oncol 12:573–583
Backes FJ, Walker CJ, Goodfellow PJ, Hade EM, Agarwal G, Mutch D, Cohn DE, Suarez AA (2016) Estrogen receptor-alpha as a predictive biomarker in endometrioid endometrial cancer. Gynecol Oncol 141:312–317
Zamagni C, Wirtz RM, De Iaco P, Rosati M, Veltrup E, Rosati F, Capizzi E, Cacciari N, Alboni C, Bernardi A, Massari F, Quercia S, D'Errico Grigioni A, Dietel M, Sehouli J, Denkert C, Martoni AA (2009) Oestrogen receptor 1 mRNA is a prognostic factor in ovarian cancer patients treated with neo-adjuvant chemotherapy: determination by array and kinetic PCR in fresh tissue biopsies. Endocr Relat Cancer 16:1241–1249
Krol MB, Galicki M, Gresner P, Wieczorek E, Jablonska E, Reszka E, Morawiec Z, Wasowicz W, Gromadzinska J (2018) The ESR1 and GPX1 gene expression level in human malignant and non-malignant breast tissues. Acta Biochim Pol 65:51–57
Aresti U, Carrera S, Iruarrizaga E, Fuente N, Marrodan I, de Lobera AR, Munoz A, Buque A, Condori E, Ugalde I, Calvo B, Vivanco GL (2014) Estrogen receptor 1 gene expression and its combination with estrogen receptor 2 or aromatase expression predicts survival in non-small cell lung cancer. PLoS ONE 9:e109659
Author information
Authors and Affiliations
Contributions
XG, MX, DM and FZ contributed to the study design. JK and YB conducted the literature search. LC and XT acquired the data. XG, MX DM, FZ and JK wrote the article and performed data analysis. All authors gave the final approval of the version to be submitted. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no potential conflicts of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, L., Cui, M., Cheng, D. et al. miR-9-5p facilitates hepatocellular carcinoma cell proliferation, migration and invasion by targeting ESR1. Mol Cell Biochem 476, 575–583 (2021). https://doi.org/10.1007/s11010-020-03927-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03927-z