Abstract
Since the first case reports in Wuhan, China, the SARS-CoV-2 has caused a pandemic and took lives of > 8,35,000 people globally. This single-stranded RNA virus uses Angiotensin-converting enzyme 2 (ACE2) as a receptor for entry into the host cell. Overexpression of ACE2 is mainly observed in hypertensive, diabetic and heart patients that make them prone to SARS-CoV-2 infection. Mitigations strategies were opted globally by the governments to minimize transmission of SARS-CoV-2 via the implementation of social distancing norms, wearing the facemasks, and spreading awareness using digital platforms. The lack of an approved drug treatment regimen, and non-availability of a vaccine, collectively posed a challenge for mankind to fight against the SARS-CoV-2 pandemic. In this scenario, repurposing of existing drugs and old treatment options like convalescent plasma therapy can be one of the potential alternatives to treat the disease. The drug repurposing provides a selection of drugs based on the scientific rationale and with a shorter cycle of clinical trials, while plasma isolated from COVID-19 recovered patients can be a good source of neutralizing antibody to provide passive immunity. In this review, we provide in-depth analysis on these two approaches currently opted all around the world to treat COVID-19 patients. For this, we used “Boolean Operators” such as AND, OR & NOT to search relevant research articles/reviews from the PUBMED for the repurposed drugs and the convalescent plasma in the COVID-19 treatment. The repurposed drugs like Chloroquine and Hydroxychloroquine, Tenofovir, Remdesivir, Ribavirin, Darunavir, Oseltamivir, Arbidol (Umifenovir), Favipiravir, Anakinra, and Baricitinib are already being used in clinical trials to treat the COVID-19 patients. These drugs have been approved for a different indication and belong to a diverse category such as anti-malarial/anti-parasitic, anti-retroviral/anti-viral, anti-cancer, or against rheumatoid arthritis. Although, the vaccine would be an ideal option for providing active immunity against the SARS-CoV-2, but considering the current situation, drug repurposing and convalescent plasma therapy and repurposed drugs are the most viable option against SARS-CoV-2.
Similar content being viewed by others
COVID-19 and SARS-CoV-2 virus
An infectious disease COVID-19 caused by a virus belongs to the Coronaviridae family was first reported in December 2019 in the Wuhan city of China. Several other lethal viruses such as Severe Acute Respiratory Syndrome-related Coronavirus (SARS-CoV) and Middle Eastern Respiratory Syndrome coronavirus (MERS-CoV) also belong to this family. The SARS-CoV is a single-stranded, enveloped positive-sense strand RNA virus with a genome size between 27 and 34 kilobases that is comparatively larger than other RNA viruses. SARS-CoV-2 driven endemic unfurled into a pandemic on 11th of March 2020 by the World Health Organization (WHO). So far, a total of seven human coronaviruses (hCoVs) types have been identified as shown in Fig. 1. The newest coronavirus strain that caused the current pandemic is known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The latest member SARS-CoV-2 has similarity close to 70% with SARS novel coronavirus. The SARS-CoV-2 infection is being characterized by severe clinical manifestations of the respiratory tract with a highly complex pathogenesis. Among targets of the virus are the epithelial cells of respiratory tract, which upon infection result to diffuse alveolar damage and severe lung injury. After entry into the cells, the virus propagates in the cytoplasm, which is also the site for formation and budding of the virus containing vesicles. The destruction of cells occurs upon release of vesicles [1].
Classification of RNA-based viruses and flow-chart showing the belongingness of Coronavirus and other closely related RNA viruses. This schematic classification of the Coronaviridae family shows how the members are divided based on sense and anti-sense strands. SARS-CoV-2 falls in category of single stranded sense strand RNA virus that is enveloped and possesses helical capsid. The α-coronaviruses are: 229E and NL63. Except SARS-CoV-2, there are other members of the β-coronavirus types are: OC43, HKU1, SARS-CoV, and MERS-CoV
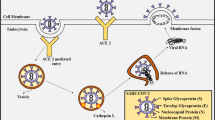
Structure of SARS-CoV-2 virus
SARS-CoV-2 RNA codes for four types of significant proteins: specific spike (S), membrane (M), nucleocapsid (N), and envelope (E) [2]. The detailed structure of the virus has been shown in the Fig. 2. The S protein is a transmembrane glycoprotein, which facilitates the virus entry into the host cell by using the signal sequence of N-terminal to have access to the endoplasmic reticulum [3]. Due to its gigantic size, it creates distinct spikes on the viral surface. The N protein helps in viral RNA synthesis, while E and M proteins are instrumental in viral assembly.
Structure of the SARS-CoV-2 virus. An RNA virus, SARS-CoV-2 consists of an envelope (E), membrane (M), spike (S), and nucleocapsid (N) proteins. The RNA is single positive-sense strand. Among those, M, S and E are glycoproteins in nature. The viral nucleo-capsid is made of proteinaceous coat capsid, inside which RNA and non-histone protein reside. SARS-CoV-2 also contains shorter spikes that possess hemagglutinin-esterase (HE) protein; their size is larger in case of Toroviruses
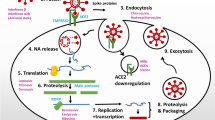
Mechanism of entry into the host cells, RAAS, and replication of SARS-CoV-2
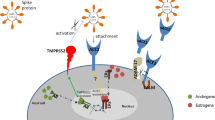
The S protein consists of two subunits: S1 & S2 by a protease Transmembrane Serine Protease 2 (TMPRSS2). TMPRSS2 is a furin-like protease that contains a single transmembrane domain and single domain for SR TRYPSIN and LDLA domains as well [4]. The gene encoding for this protein is located on 21q22.2. The primary localization of TMPRSS2 is restricted to the plasma membrane. TMPRSS2 is a secretory protein as its presence has been reported in biological fluids like semen [5] and urine [6]. The protein architecture of TMPRSS2 is shown in Fig. 3.
The priming of the S protein for pathogenicity is carried out by TMPRSS2 in coronaviruses like SARS-CoV, and MERS-CoV [7]. S protein consists of two subunits: S1 and S2. Among these, S1 subunit contains the receptor-binding domain (RBD), which binds to the SARS-CoV-2 viral receptor angiotensin-converting enzyme II (ACE2). ACE2 is a carboxypeptidase that contains one transmembrane domain and also one signal peptide [4, 8]. The gene encoding for this protein is located on Xp22. The primary localization of ACE2 is membranous and the secondary localization is extracellular. ACE2 is a secretory protein as its presence has been reported in biological fluids like plasma [5], and urine [6]. The protein architecture of ACE2 protein has been shown in Fig. 3. Further, mRNA expression levels of ACE2 in normal tissues are quite heterogeneous [9] as shown in RNAseq derived data in Fig. 4. The descending order of mRNA expression level was as follows: small intestine (93.7 ± 16.1), duodenum (69 ± 6.29), gall bladder (32.6 ± 14.37), kidney (30.8 ± 17.14), testis (26.9 ± 8.99), heart (12.3 ± 10.95), thyroid (1.39 ± 0.928), liver (1.29 ± 0.38), stomach (1.18 ± 0.937), and lung (0.345 ± 0.3). SARS-CoV-2 exploits S protein for binding to its receptors ACE2 or DPP4 (dipeptidyl peptidase 4, in bronchial epithelial cells) [10].
ACE2 Expression across major normal human organs. The RNAseq derived data shows expression of ACE2 transcript across different organs including colon, duodenum, gall bladder, heart, kidney, liver, lung, small intestine, stomach, testis and thyroid. The value of expression is shown in form of Reads Per Kilobase of transcript, per million mapped reads (RPKM), which is a normalized unit for denoting transcript expression
The mechanisms of entry and replication of SARS-CoV-2 have been shown in Fig. 5. The gene encoding for this protein is located on 2q24.3. The primary localization of CD26 is a plasma membrane and the secondary localization is extracellular. CD26 is a secretory protein as its presence has been reported in biological fluids like plasma [11], serum [12], semen [5], tears [13], and urine [14]. The protein architecture of CD26 protein has been shown in Fig. 3.
Major sites of ACE2 expression, Binding of SARS-CoV-2 to ACE2 receptor, and involvement of TMPRSS2, and DPP4 in SARS-CoV-2 entry. The spike protein (S) helps SARS-CoV-2 to enter into the host cell via binding to its receptor Angiotensin Converting Enzyme 2 (ACE2) that is part of the renin–angiotensin–aldosterone system (RAAS). RAAS and its component include angiotensinogen (AGT), the enzyme renin, angiotensin converting enzyme (ACE), and their hydrolytic products angiotensins I and II. Once SARS-CoV-2 binds to ACE2, it internalize through the process of endocytosis into the cells, which leads to downregulation of membrane-anchored ACE2. A decrease in ACE2 levels led to organ damage via activation and deactivation of ACE/Ang II/AT1R & ACE2/Ang-(1–7)/Mas-R pathways, respectively. There is alternate route of infection of SARS-CoV-2 is via transmembrane protease serine 2 (TMPRSS2) driven cleavage of SARS-CoV-2 escorted through ACE2. Due to this membrane shedding of ACE2 occurs by disintegrin and MMP17. Furthermore, soluble form of ACE2 obstructs SARS-CoV-2 from binding to membrane-anchored ACE2 in plasma membrane. An increased amount of soluble ACE2 and expression induced due to RAS inhibitors could be advantageous for protecting lungs and other organ injury but not infection with SARS-CoV-2
The N protein, which is phosphorylated, binds to SARS-CoV-2 genome are like a bead on a string fashion. The E protein is a transmembrane protein found in lower concentration and play an important role in assembly & releasing of the virus, and therefore crucial for pathogenesis. The M protein is a dimer and most abundant one among M, N, S and E protein.
Hemagglutinin-esterase (HE) exists as a dimer protein present in some beta coronaviruses. It binds to sialic acids on the surface of glycoproteins, and increase S protein-mediated viral entry into the cells, and eventually the virus spread through the mucosa. Unlike other β-coronaviruses, SARS-CoV-2 infection occurs not only in the mucosal epithelium (nasal depression and pharynx) of the upper respiratory tract but also in other organs such as of the gastric tract. Other cells types that are infected during the pathogenesis may include the neurons in brain, tubular epithelial cells of the kidneys, and intestinal mucosa cells. There have been reports of infection in sites that may lead to heart injury, failure of organs such as liver, intestine, and kidney [15].
SARS-CoV-2, cytokine storm syndrome, and organ failure
One major issue in COVID-19 cases is the blood upregulation of pro-inflammatory cytokines such as IL-1, IL6, TNF, and interferon γ. The major source of cytokine production are macrophages, as upon activation they can produce cytokines like TNF-a, interleukins including IL6, IL1, IL4, IL13, and IL18. Those further activate the cascade reaction of inflammatory factors that eventually lead to the cytokine storm syndrome (CSS), an uncontrolled response of cytokines. In CSS, an increased and uncontrolled secretion of pro-inflammatory cytokines give rises to acute respiratory distress syndrome (ARDS). It is characterized with progressive arterial hypoxemia, and breathing difficulties [16]. Respiratory failure due to ARDS is a major cause of death in COVID-19 patients [17]. CSS has been reported not only in avian H5N1 influenza virus, SARS and Middle East Respiratory Syndrome (MERS), but also in other diseases like multiple sclerosis and pancreatitis. Role of different cytokines in relation to COVID-19 has been well documented [18]. Dust cells, which are present in the alveolar region of lungs, are macrophages that play an important role in CSS. Type I IFN low levels are common to COVID-19, MERS, and SARS which could suppress Th1, but favor Th2 responses [19].
Transmission of SARS-CoV-2
Transmission of the virus can happen even from a person who shows no symptoms for COVID-19 (asymptomatic) [20]. The COVID-19 patients starts developing symptoms such as mild respiratory issues, and fever with in an incubation period between 5 and 6 days that can get extended upto 1–14 days [21]. Mode of COVID-19 transmission can be through different routes including contact, saliva, droplet, faecal and aerosol transmission [22]. Possibility of vertical transmission of COVID-19 has been also suspected, where the virus can be transmitted from parents to offspring’s via placental barrier, transcytosis of the cell-associated virus, during delivery, or through breast-feeding, but vertical transmission in case of COVID-19 was not reported until recently [23]. The first case of vertical transmission of SARS-CoV-2 in India was reported from Sassoon General Hospital, Pune, Maharashtra (India) [24].
SARS-CoV-2 transmission can happen by touching contaminated surfaces followed by nose, eyes, or mouth. To stop the transmission of SARS-CoV-2 a number of Do’s and Don'ts have been recommended by the WHO as well as by agencies like NIH and ICMR. The lists of Do's and don’ts required to mitigate COVID-19 transmission have been mentioned in the Table 1.
COVID-19 and herd immunity
When a higher percentage of the community becomes immune to a disease (that could be due to prior illness or vaccination) and makes spreading of the disease improbable is known as herd immunity. Even non-vaccinated (such as newborns and the immunocompromised one) but susceptible individuals offer immune-protection because the disease has hardly any possibility to spread within the community [39]. This is associated with the R0 (R Zero or R naught or basic reproductive number), which represents the infectivity of an infectious agent like SARS-CoV-2. The R0 value has been estimated in different studies on the SARS-CoV-2 virus ranged from 2 to 6. Between the two cohorts, the R0 observed was 2.2 [40], and 5.7, respectively [41]. In a recent study, with an R0 value of 3 for SARS-CoV-2, the threshold for herd immunity was ~ 67% which means that the decline in the incidence of SARS-CoV-2 infection will begin in the population when it surpasses 0.67 [42]. To develop herd immunity against SARS-CoV-2 there are two ways i.e. first, we vaccinate at a massive scale, but it's not possible without the availability of a safe and efficacious vaccine. The second option is via natural immunization of the world population with the virus but has seriously implication, as a large proportion of the population must be infected with SARS-CoV-2.
Here, we present a systematic review cum meta-analysis conducted to evaluate the significance of currently used treatment options for COVID-19, associated issues, and future challenges in dealing with infection and management of SARS-CoV-2. To achieve this goal, we carried out this study to evaluate the repurposing drug agents so far used for the treatment of COVID-19. For this, we used “Boolean Operators” search criteria in PUBMED to get relevant search outcome [43]. The schema for fetching the data and further filtering of the articles has been shown in Fig. 6.
Schema for screening of the articles reporting drugs repurposed for COVID-19 The NCBI search engine was searched using Boolean operators such as AND, NOT, & OR. The articles were fetched for repurposing drugs, synergism or convalescent plasma in combination with COVID-19. The articles were further segregated based on the agent used for drug repurposing
We used keywords such as:
-
I.
COVID-19 OR coronavirus = 48,139
-
II.
COVID-19 AND repurposing drugs = 234
-
III.
COVID-19 AND repurposing drugs = 232
-
IV.
COVID-19 AND repurposing drugs = 02
We have searched the literature and screened published research articles to further dig-down to list which molecule the repurposed drug targets and their route of administration whether oral, cutaneous, subcutaneous or in the form of injection, through which those have been given to the patients. Next, we corroborated the additional information by visiting the https://clinicaltrials.gov/ to get additional information on the clinical trials where the repurposed drugs have been used. The protein architecture of some of the important proteins such as ACE2, TMPRSS2, and DPP4 was extracted from the human protein reference database (HPRD) freely accessible at https://hprd.org [4]. Further, the structures of the repurposed drugs were drawn using ChemDraw Professional Version 16.0 software. The rest of the figures were made using Adobe Illustrator CS5 version 15.0.0.
There had been many treatment options adopted worldwide to treat COVID-19 patients. Among those: convalescent plasma therapy, and repurposing the drugs are taking the lead in the absence of a vaccine or unavailability of a neutralizing antibody for coronavirus.
Convalescent plasma as a potential therapy for COVID-19
The plasma derived from COVID-19 patients those successfully overcome its infection is referred to as convalescent plasma (CP). CP had been used in the past for treatment of deadly viral diseases such as Severe Acute Respiratory Syndrome (SARS), H1N1, Spanish flu, Ebola, and the MERS. The German scientist Emil von Behring got the noble prize in 1901 for the usage of CP in the treatment of diphtheria. The CP provides neutralizing antibodies to the patient against infectious agents [39]. A must to do task is to get a measurement of titer of the neutralizing antibody in advance prior to giving the plasma to the COVID-19 patients. A titer of > 1:320 must be there for the neutralizing antibody [40]. The patients who recovered from COVID-19 can be identified as potential donors if they have: (i) prior diagnosis of COVID-19, (ii) complete resolution of symptoms at least 14 days prior to donation, (iii) a negative RT-PCR result for COVID-19, and (iv) desired SARS-CoV-2 neutralizing antibody titers (optimally > 1:320). Although, the donor titer varies in current CP based trials from > 1:40 (NCT04374487 from India) to > 1:320 (USA:NCT04377672, NCT04373460, NCT04344535, Hungary:NCT04345679), the higher the better. There are more specifics coming up on the criteria for exclusion and inclusion for a donor as well as for a recipient in CP therapy. These are listed based on different clinical trials from NIH, USA as well as trials from other countries in Supplementary Table 1. Though only a handful of studies are there on the usage of CP therapy on COVID-19 patients, those have been summarized in Table 2. The exclusion and inclusion criteria for donors and acceptor have been mentioned in Supplementary Table 1.
In a small study on COVID-19 patients in Guangdong (China), after the 12th day of hospitalization of patients with severe condition CP was given. Three out of four patients discharged, and the last one was found negative using RT-PCR, two out of four patients were able to produce anti-SARS-CoV-2 IgG ~ 14 days post-transfusion [41]. A high titer antibody present in the recovered COVID-19 patients must be sufficient enough to bind SARS-CoV-2 and neutralize it to avoid access to normal cells. One of the major challenges is that CP is not used alone but in combination with other agents like corticosteroids. The neutralizing antibodies present in the CP are capable to accelerate the clearance of infected cells as well. CP constituents are capable of activating the effector mechanisms such as complement activation and phagocytosis [49]. A combination of CP and corticosteroids can reduce the viral load as well as reduce the excess of inflammatory response [47]. The initial findings from all around the world are encouraging from CP therapy supporting the evidence that the human anti-SARS-CoV-2 plasma could be able to modulate the virulence exerted by the SARS-CoV-2 via neutralization [50].
Drug repurposing as an alternative therapy for COVID-19
Drug repurposing or drug repositioning (which is sometimes also defined as drug re-profiling or drug re-tasking) is an approach for exploring the new maneuver of already approved drugs, which have been used for the treatment of other diseases [51]. In contrast, synergism is an interaction between two or more drugs that leads to overall effect to be cumulatively more than the sum of individual effect. Drug synergism is measured by calculating the combination index (CI) using freely available software CompuSyn [52]. The CI value > 1, = 1 and < 1 represents antagonistic, additive, and synergistic interaction between two or more drugs [53]. Based on the literature survey, we divided the repurposed drugs that can be used for the trials to treat COVID-19 into five categories: (I) Anti-malarial drugs (II) Drugs used for Rheumatoid arthritis (III) Cytokine modulators (IV) Protease Inhibitors (V) Others. Additionally, the details of the currently going on clinical trials are summarized in Supplementary Table 2.
The drug repurposing offers benefits in terms of time and costs required as compared to the development of a new drug from the beginning. The repurposed drugs are approved by the Food and Drug Administration (FDA), for their pharmacological properties, safety, and clinical efficacy for a different indication [54]. Therefore when used for the COVID-19 treatments, the toxicity or safety profiles of the repurposed drugs are already known. Therefore, a number of drugs have been proposed for the repurposing to treat the COVID-19 patients across the world. We are presenting basic properties of these drugs, their target and study outcome or at least observations reported in studies globally. We have summarized the information on repurposed drugs, their target, and diseases information for which those were made in Table 3.
Anti-malarial/anti-protozoan drugs
The structures of anti-malarial or anti-parasitic drugs have been shown in Fig. 7.
The chemical structure of the repurposed drugs for treatment of the COVID-19 patients. A number of drugs including anti-malarial/anti-parasitic (Chloroquine, hydroxychloroquine, and emetine), anti-myelofibrosis (Ruxolitinib), anti-viral/anti-retroviral (Tenofovir, Lopinavir, Ritonavir, Baloxavir, Remdesivir, Ribavirin, Darunavir, Oseltamivir, Arbidol, and Favipiravir), and anti-rheumatoid arthritis (Anakinra, Barcitinib, Methylprednisolone, Naproxene, and Tofacitinib) are the drugs that have been extensively in use for the treatment of COVID-19 patients
Chloroquine phosphate
Primarily chloroquine phosphate had been used for the treatment of malaria. It is a quinolone that possesses anti-inflammatory properties, and some time for amoebiasis as well. It is also known as chloroquine (CQ). CQ offers an advantage, as it does not pose complications associated with infectious complications exerted by drugs like methotrexate and leflunomide. Evidence-based on different studies showed that CQ possesses broad-spectrum anti-viral activities [80, 81]. Both anti-viral as well as anti-inflammatory activities of CQ possibly responsible for CQ’s efficacy in treating the pneumonia of COVID-19 patients [82].
Hydroxychloroquine (HCQ)
HCQ has other names/synonyms such as Oxychlorochin, Plaquenil, and Oxichloroquine. An in vitro activity against anti-SARS-CoV of HCQ was found to be superior as compared with CQ [83], and the HCQ clinical profile is also superior to CQ [84]. In terms of side effects when compared CQ with HCQ, it was found that CQ treated patients have some side effects like circular defects or Bull's eye maculopathy, retinopathy, diametric retina defects and cardiomyopathy, but patient treated with HCQ have reduced tissue accumulation that could be responsible for lesser adverse events of HCQ as compared with CQ. A high dose for > 5 years of HCQ led to retinopathy development, which is in concordance with the HCQ as a therapy [85, 86].
Emetine
Emetine is an alkaloid isolated from the flowering plant Carapichea ipecacuanha a member of the family Rubiaceae. Emetine has been used against protozoan infections and also to induce vomiting. Emetine is a translation-inhibiting drug that had been used in amoebiasis treatment. It is capable of inhibiting translation machinery of the malaria parasite (Plasmodium falciparum) by binding to the E ribosomal site. This shows anti-viral activity against a wide range of viruses (both DNA and RNA based) including Zika, rabies, cytomegalovirus, Ebola, and HIV-1 virus. Emetine also showed anti-viral activity against hCoV-OC43, SARS-CoV, hCoV-NL43, MHV-A59, and MERS-CoV in an in vitro condition. The viral polymerase enzyme and some host proteins are the targets of Emetine [18]. It has been recently reported that emetine inhibits the replication of SARS-CoV-2 at ~ 0.5 μM concentration. The in vivo achievable concentration of emetine in plasma is 0.075 μg/mL (0.156 μM), lower than the in vitro EC50 against SARS-CoV-2 [19].
Anti-viral drugs
These drugs work against different viruses including retroviruses like HIV-1 and have been proposed to use for the treatment of COVID-19. The structures of selected anti-retroviral/anti-viral drugs have been shown in Fig. 7.
Favipiravir
Favipiravir is an oral anti-viral drug used for the treatment of influenza. It came into limelight for Ebola treatment during the 2014 epidemic in West Africa as there was no standard of care (SOC) was available. Effectiveness of favipiravir was also observed for prophylaxis and infectious animal models of lethal Ebola virus [87]. It is a purine analogue and also known as 6-fluoro-3-hydroxy-2-pyrazinecarboxamide or T-705 or Avigan as a brand name, which targets viral RdRp (RNA dependent RNA Polymerase). On an urgent basis, favipiravir had been approved for the clinical trial in adult COVID-19 patient’s treatment (2020L00005). SARS-CoV-2 also possess RdRp gene similar to other members of the family (SARS-CoV and MERS-CoV), which makes favipiravir eligible to be tested against SARS-CoV-2 virus. It is a pro-drug which upon ribosylation and phosphorylation form an active metabolite intracellularly called T-705RTP or favipiravir ibofuranosyl-5′-triphosphate (T-705RTP), which interfere with the replication of the virus by competing with the naturally occurring purine nucleosides and inhibits the viral RdRp of SARS-CoV-2.
In Shenzhen (China), a clinical trial of favipiravir on COVID-19 patients was conducted for evaluation of safety and efficacy (ChiCTR2000029600). A total of 35 patients in the favipiravir arm showed significantly a shorter viral clearance duration in contrast with the control arm containing 45 patients. Further, these findings were corroborated with chest X-rays showing improvement in the favipiravir arm (91.43% vs 62%) [88]. In another multi-centric randomized study (ChiCTR200030254), favipiravir treatment of COVID-19 patients led to an improved recovery at 7th day from 55.86 to 71.43% [89].
Remdesivir
Remdesivir (also known as GS-5734) is a 1′-cyano-substituted adenosine analogue. It is a pro-drug that inhibits viral RNA polymerases, has shown in vitro activity against coronaviruses like SARS-CoV-2, CoV-229E, SARS-CoV, CoV-OC43, and MERS-CoV [90]. It is a mono phosphoramidate pro-drug possessing wide anti-viral spectrum covering filoviruses, coronaviruses, pneumoviruses, and paramyxoviruses. It has been observed that remdesivir inhibits humans as well as animal coronaviruses in vitro, including SARS-CoV-2. Remdesivir proved to be a superior drug in a lethal murine MERS model as compared with a regimen of IFN-b, and lopinavir-ritonavir combination. An EC50 of remdesivir was 0.77 μM against SARS-CoV-2 virus [91]. It has been documented mutations such as F476L and V553L in the nsp12 polymerase gene of murine hepatitis virus confer remdesivir resistance [92].
Alovudine
Alovudine (also known as fluorothymidine) a DNA polymerase inhibitor developed by Medivir is an anti-viral agent. Due to toxicity issues, in 2005 after phase II clinical trial, it was discontinued. Alovudine is a nucleoside reverse transcriptase inhibitor analog of thymidine [93]. Alovudine is able to terminate the RNA synthesis SARS-CoV-2 virus, but more work is required before it makes an entry into a clinical trial.
Drug used for rheumatoid arthritis
The structures of drugs used for rheumatoid arthritis, but now repurposed for treating the COVID-19 patients are shown in Fig. 7.
Baricitinib
Baricitinib is an orally available agent used for rheumatoid arthritis. It is sold with the brand name Olumiant. It inhibits the response of inflammatory molecules and cytokine production via modulation of JAK-STAT pathway [94]. It is an active ingredient of olumiant. Baricitinib is a reversible inhibitor of JAK1/JAK2. According to the EU Clinical Trials Register there are already phase-II, and III (2020–001854-23), and phase-IV (2020–001354-22) clinical trials using Baricitinib on COVID-19 patients. Modulation of cytokine dysregulation could affect the host inflammatory response and entry of viruses into the cells. This makes it an ideal agent to be tested in COVID-19 patients [95, 96].
Tofacitinib (oral)
Baricitinib and tofacitinib are first-generation JAK inhibitors. Tofacitinib is a small molecule inhibitor of Janus Kinases particularly JAK1/JAK3 [97]. It is sold with the brand name Xeljanz. It had been used for the treatment of RA (moderate to severe form). Tofacitinib is N-acylpiperidine compound. Tofacitinib inhibits STAT also, but in a reversible manner. Tofacitinib subjects to hepatic metabolism through cytochrome CYP3A4 mainly which means a combination of CYP3A4 inhibitors could be tested first in vitro to see if there is a synergistic impact [98].
Ruxolitinib (oral)
It has been used for the treatment of moderate to high-risk myelofibrosis. It is sold in the market with the trade name Jakafi or Jakavi. Ruxolitinib is an oral kinase inhibitor that inhibits JAK1 and JAK2. It is also known as INCB01842. Metabolism of ruxolitinib is facilitated by CYP3A4. The chemical constituent of Ruxolitinib belongs to the pyrrolo [2, 3-d] pyrimidines class of organic compound.
Cytokine modulators
Tocilizumab
An anti-IL6 blocker targets the IL6 receptor proved to be effective in rheumatoid arthritis treatment [99], and later for juvenile idiopathic arthritis [100], giant cell arteritis [101]. IL6 is a bonafide marker for inflammation. It is also known by another famous name Actemra and recently approved by the FDA for testing in a clinical trial for COVID-19 patients. It is a recombinant antibody humanized and of IgG1 class. Actemra is capable of disrupting inflammatory response exerted by IL6 is known as cytokine release syndrome (CRS). The efficacy of Actemra was tested on COVID-19 patients at The First Affiliated Hospital of the University of Science and Technology, China. Among 21 patients tested, the body temperature returns to normal in all the cases. An improvement in respiratory function was seen in 100% of the patients and the recovery rate was ~ 95% as seen in CT scan reports, and the patients were discharged within 14 days of post-tocilizumab treatment. The findings extrapolated on 500 severe or critical patients enrolled in a clinical trial (ChiCTR2000029765) [102]. In contrast, the Italian guidelines suggest that tocilizumab use is suitable in patients with major symptoms including when high viral load is over, and patients don’t have any fever (Apyretic) for > 72 h or 7 days post-onset of symptoms, and increased IL6 levels [94].
Anakinra
Anakinra is a recombinant human antagonist of IL1R that has been used in rheumatoid arthritis. These drugs also play an important role in the management of CRS. Due to the release of IL1R SARS-CoV-2 causes an advanced form of cell death occurs due to inflammation (pyroptosis) and mediated by caspase-1. The repurposed drug anakinra in case of COVID-19 patients in phase III randomized clinical trial able to reduce both requirement of invasive mechanical ventilation in ICU as well as the mortality rate in severe COVID-19 cases without serious side-effects [79].
Adalimumab (anti-TNF-α agent)
It has been earlier used for treatment of Rheumatoid arthritis. The mode of adalimumab for patients is subcutaneous. FDA approved it long back in 2002 for treatment of RH. Biosimilar of adalimumab (Hyrimoz) is also available which is available by the name adalimumab-adaz approved in Oct 2018 by the FDA. Adalimumab binds to TNFα and leads to inhibition of interaction with the receptor of TNF by binding with p55 & p75. There is a trial going on (ChiCTR2000030089) where one arm includes conventional treatment along with adalimumab [103]. An interesting observation has been mentioned regarding levels TNF-α that it was moderately high in SARS but significantly higher levels in COVID-19 patients.
Protease inhibitors anti-retroviral/anti-viral drugs
Lopinavir
Lopinavir is an anti-retroviral drug used for the treatment of HIV patients. It is a protease inhibitor, which has been used for the treatment of SARS-CoV infected patients in combination with ritonavir & ribavirin in a non-randomized clinical trial. Only few SARS patients progressed to ARDS with this treatment as compared with patients receiving only ribavirin and corticosteroids. It was sold by the brand name Kaletra. It is interesting to note that Lopinavir is exclusively given along with ritonavir because lopinavir possesses poor oral bioavailability and extensive biotransformation. On the other hand, Ritonavir is an inhibitor of the enzymes related to lopinavir metabolism, therefore a co-administration boosts the lopinavir exposure and significantly improves the anti-viral activity [104]. Finding of a randomized control trial (ChiCTR2000029308) on SARS-CoV-2 showed no significant benefit of lopinavir-ritonavir combination in SARS-CoV-2 patients as compared with SOC [105]. Combining lopinavir with other agents to treat SARS-CoV-2 virus not only increased synergism but also decreased the lopinavir inhibitory concentration.
Ritonavir
It is another anti-retroviral drug used against HIV. It's a protease inhibitor that inhibits the productive cycle of the HIV virus. It is sold with the trade name Norvir. Ritonavir inhibits HIV-1 protease as well as host's cytochrome P450 3A4 enzyme that helps in metabolizing lopinavir. The NIH panel recommended not using the combination of lopinavir/ritonavir or other HIV protease inhibitors due to unfavorable outcome post-treatment of these agents.
Ribavirin
Ribavirin or tribavirin is an anti-viral drug used for Rous sarcoma virus infection, viral hemorrhagic fevers and hepatitis C. Ribavirin is synthetic guanosine nucleoside that interferes with the viral mRNA synthesis. Ribavirin has been used in combination with interferon beta-1b, lopinavir-ritonavir in a randomized phase-II clinical trial in COVID-19 patients and the early results showed that it was superior to alone lopinavir-ritonavir combination in reducing the symptoms exerted by the virus and shortening shedding of the virus [106].
Camostat mesilate
Camostat mesilate (CM) is an inhibitor of TMPRSS2. It inhibits the serine proteases like TMPRSS2 [7]. CM proved to be effective in blocking the spreading and virulence of SARS-CoV in a lethal mouse model [107]. It was observed that CM is capable of blocking the entry of SARS-CoV-2 into the lung cells. Camostat inhibits diverse range of proteases including plasmin, trypsin, kallikrein and thrombin [108]. University of Tokyo, Japan planned to conduct a clinical trial on the combination of CM and nafamostat on COVID-19 patients. CM was approved in Japan for treatment of pancreatic inflammation [109].
Homoharringtonine
Homoharringtonine is also known as omacetaxine mepesuccinate or HHT. HHT is a cephalotaxine ester. It was isolated from the leave of Cephalotaxus fortunei of family Taxaceae. Omacetaxine received orphan drug status from FDA in March 2006 (according to FDA an orphan drug is the one which is intended to treat a rare disease which affect < 200,000 persons in the United States) for treatment of chronic myeloid leukemia patients particularly those who found to be resistant to > 2 tyrosine kinase inhibitors. HHT shows anti-cancer activity via inhibition of translation by binding to ribosomal site-A. This forces the cells to lose proteins like MCL1 and c-MYC (both with short half-life), crucial for leukemia cell’s survival. HHT showed activity against a large number of viruses including pseudorabies virus, rabies virus, hepatitis B virus, Newcastle disease virus, and echovirus 1 [110]. In an in vitro screening in Vero E6 cells, HHT inhibited SARS-CoV-2 replication at an EC50 of < 100 μM [111].
An urgent requirement and challenges for more treatment options for COVID-19
Availability of safe and efficacious vaccine against COVID-19
Multiple pharmaceutical companies and academic institutions are joining the hands for the collaborative efforts to develop a vaccine against SARS-CoV-2. There are multiple candidates proposed for the potential vaccine against SARS-CoV-2 that include mRNA vaccine, inactivated virus vaccine, DNA vaccine recombinant protein vaccine, and viral vector-based vaccine. Globally, the experts in vaccine development think that it will take around 18 months to develop a SARS-CoV-2 vaccine, although it is very optimistic even if we consider this to be the fastest created new vaccine in the history. In traditional settings, it takes 5 to 6 years to develop a vaccine, but the high mutation rate of RNA viruses and therefore changing the specific immune response make it even harder to develop an efficacious vaccine. Every vaccine in human clinical trials goes through three phases: Phase-I is the safety trial in a small group of healthy volunteers, where a vaccine is tried out with different dosages to find out the strongest immune response without serious side effects. The phase-II vaccine trials test how well the vaccine works in hundreds of people of diverse age and health status. Next, in phase three, the vaccine is given to thousands of people who are already at the risk of infection, and then wait to see if the vaccine reduces the number of people getting sick. Since phase-III is tried out in natural disease condition and larger population size, it is usually the longest phase. A number of potential candidates for vaccines are in pre-clinical studies and a few in clinical trials in different parts of the world including the USA, Russia, China, UK, and India. Regardless which of these countries get success and finally a vaccine gets approved, the first challenge is whether the country is willing to share it with the rest of the world, and a proper storage and distribution system. So, overcoming these challenges requires close collaboration between pharma giants, regulatory bodies like FDA, active involvement and cooperation of the scientific community, and healthcare systems.
Development of antibodies neutralizing the virus
Most of the anti-SARS-CoV novel antibodies (nAbs) have been targeted against S protein, RBD [112], S2 subunit, and S1/S2 proteolytic cleavage sites. Some nAbs like S230.15, m396, S109.8 and S227.14 showed neutralizing activity against human, raccoon dog, and palm civet but none of these have been evaluated in clinical studies. Antibodies like 80R (scFv or mAb) is capable of neutralizing the infection of SARS-CoV via blockage of RBD-ACE2 interaction [113]. There is a high sequence similarity for S protein between SARS-CoV-2 and SARS-CoV [114]. This suggests a cross neutralizing/cross-reactivity of nAbs between SARS-CoV and SARS-CoV-2 infection. The SARS-CoV mAB CR3022 (RBD-specific) could possibly bind to RBD of SARS-CoV-2 because RBD of SARS-CoV & SARS-CoV-2 are very similar [115]. Different fragments like S1-NTD, S2, and RBD have been used as a target for the development of nAbs. A similar strategy could be adopted for SARS-CoV-2. CP is currently in use for the treatment of COVID-19 patients, but non-nAbs targeting other regions than RBD of S-protein can create antibody-dependent enhancement (ADE) effect on virulence as well as on the disease [116].
Mesenchymal stem cell therapy for COVID-19
Stem cell therapy proved to be very useful in treating a number of diseases including cancer [117], and diabetes [118]. Mesenchymal stem cells (MSC) are characterized by low invasive nature and high proliferation rate, and additionally devoid of ethical & social issues that makes it as the preferred therapeutic option over others [119]. MSCs play an important role in immunomodulatory effects via secreting many types of cytokines by paracrine secretion or make direct interactions with immune cells. The source of MSCs can be peripheral blood (PB), bone marrow (BM), adipose tissues [(AT), buccal fat pad, abdominal fat, & infrapatellar fat pad], placenta, umbilical cord, Warton jelly, amniotic fluid, and blood cord. Therefore, it seems MSCs-based therapy may possibly be an ideal candidate for clinical trials or at least the combination of treatment to treat COVID-19 patients.
MSC therapy was applied in COVID-19 patients on seven patients. The levels of peripheral lymphocyte were increased, and on day 6 cytokine secreting cells (CXCR3+ CD4+ T, CXCR3+ CD8+ T, and NK CXCR3+ cells) were disappeared. The Dendritic cell population and IL10 were increased, but TNF-α level was decreased. The MSCs were found to be negative for ACE2 and TMPRSS2 suggesting there was no SARS-CoV-2 infection [120].
In addition, recently a case study was reported in China on a female patient with an acute COVID-19 syndrome that the results of laboratory tests and CT images provided extremely effective results after 21 days of treatment with umbilical cord MSCs. Upon treatment with MSCs, an increase in lymphocyte and a decrease both in WBCs and neutrophils was observed. Some T cell surface markers like CD3, CD4, and CD8 were increased. CT scans showed that the pneumonia was cleared [121]. Including the ground-glass opacity in the lung, the other typical diagnosis characteristic of critically ill patients was a significant decrease in lymphocytes along with the increase of neutrophils. However, only a handful studies on MSCs show promising results in the treatment of COVID-19 patients. Though only on a limited number of patients, but these studies showed that MSC therapy alone or in combination with other drugs can be used to treat SARS-CoV-2 infected patients.
An unobvious challenge of vaccine nationalism
During COVID-19 pandemic one issue emerged where different countries particularly the USA, Russia, and China are in the race of making a vaccine against SARS-CoV-2. At the same time, countries like USA and Russia are securing a large and sufficient number of vaccine doses against COVID-19 for their own people and prioritizing their own market rather than making available to other countries. This is popularly known as ‘vaccine nationalism’. This can be executed through pre-purchase agreements between a vaccine manufacturer and the government. WHO issued a warning regarding vaccine nationalism as instead of helping mankind, it’s going to help the virus. It’s not a new challenge, as a similar scenario was observed during The H1N1 flu pandemic in 2009. At that time, among vaccine producers for H1N1 flu Australia was the leader and the government blocked the exports, but at the same time, the rich countries went for the pre-purchase agreements with some pharma giants. In the case of COVID-19, US government already has shown interest to secure 600,000 doses. Though vaccine nationalism is against the principles of global public health, unfortunately, there is no law to prohibit the pre-purchase in pandemic like COVID-19.
Conclusion
The existence of SARS-CoV-2 was reported in 2019. Since then it posed a threat to mankind around the world. Hastening of treatment options for COVID-19 brought nothing so far but we have to look at old treatment option as a savior because we know convalescent plasma therapy and repurposing drugs approaches had been used in the past in crisis period. While massive-scale efforts to make a suitable vaccine are on the way, time being number of drugs used for other diseases have been currently repurposed to tackle the COVID-19 pandemic.
Globally, the experts in vaccine development are optimistic to deliver the vaccine in next 12 to 18 months but the time frame may vary because from the selection of the suitable target to testing in the animals and then different phases of clinical trials are time consuming processes and require quality controls. Though, FDA has put vaccine development and their approval on the fast track, but there are no short cuts for different stages of developments and quality controls directly associated with the safety and efficacy. There are already concerns raised by different scientists including Dr Anthony Fauchi about the COVID-19 vaccine from Russia. With all the hopes on the potential vaccine’s progress, the strategies are also required to build the infrastructure for equitable distribution of the vaccine and to avoid bottleneck on the availability as soon as the vaccine successfully completes the clinical trials. While we wait for a vaccine to come into the picture, convalescent plasma therapy, and repurposing the drugs treatment options proved to be suitable (if not perfect). We need to have suitable vaccine development, neutralizing nABs antibody as prophylactic and therapeutic, and mesenchymal stem cell-based treatment options for effectively dealing with COVID-19. Until an ideal treatment comes, people must follow proper caution such as wearing masks, follow social distancing, and as much as possible do activities, which could be afforded through online mode/route.
Data availability
All data generated and analyzed during our study are included in the published article and also supplied in two supplementary tables.
References
Kahn JS, McIntosh K (2005) History and recent advances in coronavirus discovery. Pediatr Infect Dis J 24:S223–S227. https://doi.org/10.1097/01.inf.0000188166.17324.60(discussion S226)
Song Z, Xu Y, Bao L, Zhang L, Yu P, Qu Y, Zhu H, Zhao W, Han Y, Qin C (2019) From SARS to MERS thrusting coronaviruses into the spotlight. Viruses 11:59. https://doi.org/10.3390/v11010059
Raj K, Rohit GA, Singh S (2020) Coronavirus as silent killer: recent advancement to pathogenesis, therapeutic strategy and future perspectives. VirusDisease 151:424–437. https://doi.org/10.1007/s13337-020-00580-4
Keshava Prasad TS, Goel R, Kandasamy K, Keerthikumar S, Kumar S, Mathivanan S, Telikicherla D, Raju R, Shafreen B, Venugopal A, Balakrishnan L, Marimuthu A, Banerjee S, Somanathan DS, Sebastian A, Rani S, Ray S, Harrys Kishore CJ, Kanth S, Ahmed M, Kashyap MK, Mohmood R, Ramachandra YL, Krishna V, Rahiman BA, Mohan S, Ranganathan P, Ramabadran S, Chaerkady R, Pandey ACP (2009) Human protein reference database–2009 update. Nucleic Acids Res 37:D767–D772
Pilch B, Mann M (2006) Large-scale and high-confidence proteomic analysis of human seminal plasma. Genome Biol 7:R40. https://doi.org/10.1186/gb-2006-7-5-r40
Adachi J, Kumar C, Zhang Y, Olsen JV, Mann M (2006) The human urinary proteome contains more than 1500 proteins, including a large proportion of membrane proteins. Genome Biol 7:R80. https://doi.org/10.1186/gb-2006-7-9-R80
Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Muller MA, Drosten C, Pohlmann S (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181:e8. https://doi.org/10.1016/j.cell.2020.02.052
Rao R, Husain A, Bharti AC, Kashyap MK (2019) Discovery of a novel connecting link between renin-angiotensin system and cancer in Barrett's esophagus by proteomic screening. Proteom Clin Appl 13:e1900006. https://doi.org/10.1002/prca.201900006
Fagerberg L, Hallstrom BM, Oksvold P, Kampf C, Djureinovic D, Odeberg J, Habuka M, Tahmasebpoor S, Danielsson A, Edlund K, Asplund A, Sjostedt E, Lundberg E, Szigyarto CA, Skogs M, Takanen JO, Berling H, Tegel H, Mulder J, Nilsson P, Schwenk JM, Lindskog C, Danielsson F, Mardinoglu A, Sivertsson A, von Feilitzen K, Forsberg M, Zwahlen M, Olsson I, Navani S, Huss M, Nielsen J, Ponten F, Uhlen M (2014) Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteom 13:397–406. https://doi.org/10.1074/mcp.M113.035600
Dalan R (2020) Is DPP4 inhibition a comrade or adversary in COVID-19 infection. Diabetes Res Clin Pract 164:108216. https://doi.org/10.1016/j.diabres.2020.108216
Sedo A, Malik R, Vicar J, Simanek V, Ulrichova J (2003) Quaternary benzo[c]phenanthridine alkaloids as inhibitors of dipeptidyl peptidase IV-like activity baring enzymes in human blood plasma and glioma cell lines. Physiol Res 52:367–372
Iwaki-Egawa S, Watanabe Y, Kikuya Y, Fujimoto Y (1998) Dipeptidyl peptidase IV from human serum: purification, characterization, and N-terminal amino acid sequence. J Biochem 124:428–433. https://doi.org/10.1093/oxfordjournals.jbchem.a022130
de Souza GA, Godoy LM, Mann M (2006) Identification of 491 proteins in the tear fluid proteome reveals a large number of proteases and protease inhibitors. Genome Biol 7:R72. https://doi.org/10.1186/gb-2006-7-8-R72
Khan A, Packer NH (2006) Simple urinary sample preparation for proteomic analysis. J Proteome Res 5:2824–2838. https://doi.org/10.1021/pr060305y
Yang HY, Duan GC (2020). [Analysis on the epidemic factors for COVID-19]. Zhonghua Yu Fang Yi Xue Za Zhi 54:608-613. Chinese. https://doi.org/10.3760/cma.j.cn112150-20200227-00196
Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD (2005) Incidence and outcomes of acute lung injury. N Engl J Med 353:1685–1693. https://doi.org/10.1056/NEJMoa050333
Gibson PG, Qin L, Puah SH (2020) COVID-19 acute respiratory distress syndrome (ARDS): clinical features and differences from typical pre-COVID-19 ARDS. Med J Aust 213:e1. https://doi.org/10.5694/mja2.50674
Feldmann M, Maini RN, Woody JN, Holgate ST, Winter G, Rowland M, Richards D, Hussell T (2020) Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet 395:1407–1409. https://doi.org/10.1016/S0140-6736(20)30858-8
Torres T, Puig L (2020) Managing cutaneous immune-mediated diseases during the COVID-19 pandemic. Am J Clin Dermatol 21:307–311. https://doi.org/10.1007/s40257-020-00514-2
Liu Z, Magal P, Seydi O, Webb G (2020) A COVID-19 epidemic model with latency period. Infect Dis Model https://doi.org/10.1016/j.idm.2020.03.003
Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, Azman AS, Reich NG, Lessler J (2020) The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med 172:577–582. https://doi.org/10.7326/M20-0504
McDermott CV, Alicic RZ, Harden N, Cox EJ, Scanlan JM (2020) Put a lid on it: are faecal bio-aerosols a route of transmission for SARS-CoV-2? J Hosp Infect https://doi.org/10.1016/j.jhin.2020.04.024
Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, Li J, Zhao D, Xu D, Gong Q, Liao J, Yang H, Hou W, Zhang Y (2020) Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet 395:809–815. https://doi.org/10.1016/S0140-6736(20)30360-3
Kulkarni R, Rajput U, Dawre R, Valvi C, Nagpal R, Magdum N, Vankar H, Sonkawade N, Das A, Vartak S, Joshi S, Varma S, Karyakarte R, Bhosale R, Kinikar A (2020) Early-onset symptomatic neonatal COVID-19 infection with high probability of vertical transmission. Infection 1–5. https://doi.org/10.1007/s15010-020-01493-6
Brake SJ, Barnsley K, Lu W, McAlinden KD, Eapen MS, Sohal SS (2020) Smoking upregulates angiotensin-converting enzyme-2 receptor: a potential adhesion site for novel coronavirus SARS-CoV-2 (Covid-19). J Clin Med. https://doi.org/10.3390/jcm9030841
Giwa AL, Desai A, Duca A (2020) Novel 2019 coronavirus SARS-CoV-2 (COVID-19): an updated overview for emergency clinicians. Emerg Med Pract 22:1–28
Tirupathi R, Bharathidasan K, Palabindala V, Salim SA, Al-Tawfiq JA (2020) Comprehensive review of mask utility and challenges during the COVID-19 pandemic. Infez Med 28:57–63
Ozma MA, Maroufi P, Khodadadi E, Kose S, Esposito I, Ganbarov K, Dao S, Esposito S, Dal T, Zeinalzadeh E, Kafil HS (2020) Clinical manifestation, diagnosis, prevention and control of SARS-CoV-2 (COVID-19) during the outbreak period. Infez Med 28:153–165
Berardi A, Perinelli DR, Merchant HA, Bisharat L, Basheti IA, Bonacucina G, Cespi M, Palmieri GF (2020) Hand sanitisers amid CoViD-19: a critical review of alcohol-based products on the market and formulation approaches to respond to increasing demand. Int J Pharm 584:119431. https://doi.org/10.1016/j.ijpharm.2020.119431
MacIntyre CR, Wang Q (2020) Physical distancing, face masks, and eye protection for prevention of COVID-19. Lancet 395:1950–1951. https://doi.org/10.1016/S0140-6736(20)31183-1
Wilder-Smith A, Freedman DO (2020) Isolation, quarantine, social distancing and community containment: pivotal role for old-style public health measures in the novel coronavirus (2019-nCoV) outbreak. J Travel Med https://doi.org/10.1093/jtm/taaa020
West R, Michie S, Rubin GJ, Amlot R (2020) Applying principles of behaviour change to reduce SARS-CoV-2 transmission. Nat Hum Behav 4:451–459. https://doi.org/10.1038/s41562-020-0887-9
Santos C, Kieszak S, Wang A, Law R, Schier J, Wolkin A (2017) Reported adverse health effects in children from ingestion of alcohol-based hand sanitizers—United States, 2011–2014. MMWR Morb Mortal Wkly Rep 66:223–226. https://doi.org/10.15585/mmwr.mm6608a5
Muller O, Neuhann F, Razum O (2020) Epidemiology and control of COVID-19. Dtsch Med Wochenschr 145:670–674. https://doi.org/10.1055/a-1162-1987
Adhikari SP, Meng S, Wu YJ, Mao YP, Ye RX, Wang QZ, Sun C, Sylvia S, Rozelle S, Raat H, Zhou H (2020) Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty 9:29. https://doi.org/10.1186/s40249-020-00646-x
Kulkarni P, Kodad S, Mahadevappa M (2020) Covid-19 and Namaste. Influenza Other Respir Viruses. https://doi.org/10.1111/irv.12746
Ming LC, Untong N, Aliudin NA, Osili N, Kifli N, Tan CS, Goh KW, Ng PW, Al-Worafi YM, Lee KS, Goh HP (2020) Mobile health apps on COVID-19 launched in the early days of the pandemic: content analysis and review. JMIR Mhealth Uhealth. https://doi.org/10.2196/19796
Collado-Borrell R, Escudero-Vilaplana V, Villanueva-Bueno C, Herranz-Alonso A, Sanjurjo-Saez M (2020) Features and functionalities of smartphone apps related to COVID-19. J Med Internet Res. https://doi.org/10.2196/20334
Marson P, Cozza A, De Silvestro G (2020) The true historical origin of convalescent plasma therapy. Transfus Apher Sci. https://doi.org/10.1016/j.transci.2020.102847
Bloch EM, Shoham S, Casadevall A, Sachais BS, Shaz B, Winters JL, van Buskirk C, Grossman BJ, Joyner M, Henderson JP, Pekosz A, Lau B, Wesolowski A, Katz L, Shan H, Auwaerter PG, Thomas D, Sullivan DJ, Paneth N, Gehrie E, Spitalnik S, Hod E, Pollack L, Nicholson WT, Pirofski LA, Bailey JA, Tobian AA (2020) Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest 130:2757–2765. https://doi.org/10.1172/JCI138745
Zhang B, Liu S, Tan T, Huang W, Dong Y, Chen L, Chen Q, Zhang L, Zhong Q, Zhang X, Zou Y, Zhang S (2020) Treatment with convalescent plasma for critically Ill Patients with severe acute respiratory syndrome coronavirus 2 infection. Chest 158:e9–e13. https://doi.org/10.1016/j.chest.2020.03.039
Randolph HE, Barreiro LB (2020) Herd immunity: understanding COVID-19. Immunity 52:737–741. https://doi.org/10.1016/j.immuni.2020.04.012
Tungekar A, Mandarthi S, Mandaviya PR, Gadekar VP, Tantry A, Kotian S, Reddy J, Prabha D, Bhat S, Sahay S, Mascarenhas R, Badkillaya RR, Nagasampige MK, Yelnadu M, Pawar H, Hebbar P, Kashyap MK (2018) ESCC ATLAS: a population wide compendium of biomarkers for esophageal squamous cell carcinoma. Sci Rep 8:12715. https://doi.org/10.1038/s41598-018-30579-3
Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, Wang F, Li D, Yang M, Xing L, Wei J, Xiao H, Yang Y, Qu J, Qing L, Chen L, Xu Z, Peng L, Li Y, Zheng H, Chen F, Huang K, Jiang Y, Liu D, Zhang Z, Liu Y, Liu L (2020) Treatment of 5 critically Ill patients with COVID-19 with convalescent plasma. JAMA. https://doi.org/10.1001/jama.2020.4783
Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, Zhou M, Chen L, Meng S, Hu Y, Peng C, Yuan M, Huang J, Wang Z, Yu J, Gao X, Wang D, Yu X, Li L, Zhang J, Wu X, Li B, Xu Y, Chen W, Peng Y, Hu Y, Lin L, Liu X, Huang S, Zhou Z, Zhang L, Wang Y, Zhang Z, Deng K, Xia Z, Gong Q, Zhang W, Zheng X, Liu Y, Yang H, Zhou D, Yu D, Hou J, Shi Z, Chen S, Chen Z, Zhang X, Yang X (2020) Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci USA 117:9490–9496. https://doi.org/10.1073/pnas.2004168117
Ye M, Fu D, Ren Y, Wang F, Wang D, Zhang F, Xia X, Lv T (2020) Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J Med Virol. https://doi.org/10.1002/jmv.25882
Ahn JY, Sohn Y, Lee SH, Cho Y, Hyun JH, Baek YJ, Jeong SJ, Kim JH, Ku NS, Yeom JS, Roh J, Ahn MY, Chin BS, Kim YS, Lee H, Yong D, Kim HO, Kim S, Choi JY (2020) Use of convalescent plasma therapy in two COVID-19 patients with acute respiratory distress syndrome in Korea. J Korean Med Sci 35:e149. https://doi.org/10.3346/jkms.2020.35.e149
Perotti C, Baldanti F, Bruno R, Del Fante C, Seminari E, Casari S, Percivalle E, Glingani C, Musella V, Belliato M, Garuti M, Meloni F, Frigato M, Di Sabatino A, Klersy C, De Donno G, Franchini M, Covid-19 plasma task f (2020) Mortality reduction in 46 severe Covid-19 patients treated with hyperimmune plasma. A proof of concept single arm multicenter trial. Haematologica. https://doi.org/10.3324/haematol.2020.261784
Cunningham AC, Goh HP, Koh D (2020) Treatment of COVID-19: old tricks for new challenges. Crit Care 24:91. https://doi.org/10.1186/s13054-020-2818-6
Teixeira da Silva JA (2020) Convalescent plasma: a possible treatment of COVID-19 in India. Med J Armed Forces India 76:236–237. https://doi.org/10.1016/j.mjafi.2020.04.006
Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A, Doig A, Guilliams T, Latimer J, McNamee C, Norris A, Sanseau P, Cavalla D, Pirmohamed M (2019) Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov 18:41–58. https://doi.org/10.1038/nrd.2018.168
Chou TC (2010) Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res 70:440–446. https://doi.org/10.1158/0008-5472.CAN-09-1947
Kashyap MK, Amaya-Chanaga CI, Kumar D, Simmons B, Huser N, Gu Y, Hallin M, Lindquist K, Yafawi R, Choi MY, Amine AA, Rassenti LZ, Zhang C, Liu SH, Smeal T, Fantin VR, Kipps TJ, Pernasetti F, Castro JE (2017) Targeting the CXCR4 pathway using a novel anti-CXCR4 IgG1 antibody (PF-06747143) in chronic lymphocytic leukemia. J Hematol Oncol 10:112. https://doi.org/10.1186/s13045-017-0435-x
Khalili JS, Zhu H, Mak NSA, Yan Y, Zhu Y (2020) Novel coronavirus treatment with ribavirin: groundwork for an evaluation concerning COVID-19. J Med Virol 92:740-746. https://doi.org/10.1002/jmv.25798
Kuriya B, Cohen MD, Keystone E (2017) Baricitinib in rheumatoid arthritis: evidence-to-date and clinical potential. Ther Adv Musculoskelet Dis 9:37–44. https://doi.org/10.1177/1759720X16687481
Furuta Y, Komeno T, Nakamura T (2017) Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B 93:449–463. https://doi.org/10.2183/pjab.93.027
Sheahan TP, Sims AC, Zhou S, Graham RL, Pruijssers AJ, Agostini ML, Leist SR, Schafer A, Dinnon KH 3rd, Stevens LJ, Chappell JD, Lu X, Hughes TM, George AS, Hill CS, Montgomery SA, Brown AJ, Bluemling GR, Natchus MG, Saindane M, Kolykhalov AA, Painter G, Harcourt J, Tamin A, Thornburg NJ, Swanstrom R, Denison MR, Baric RS (2020) An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci Transl Med 12(541):eabb5883. https://doi.org/10.1126/scitranslmed.abb5883
de Oliveira JT, Santos AL, Gomes C, Barros R, Ribeiro C, Mendes N, de Matos AJ, Vasconcelos MH, Oliveira MJ, Reis CA, Gartner F (2015) Anti-influenza neuraminidase inhibitor oseltamivir phosphate induces canine mammary cancer cell aggressiveness. PLoS ONE 10:e0121590. https://doi.org/10.1371/journal.pone.0121590
Eastman RT, Roth JS, Brimacombe KR, Simeonov A, Shen M, Patnaik S, Hall MD (2020) Remdesivir: a review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS Cent Sci 6:672–683. https://doi.org/10.1021/acscentsci.0c00489
Liu J, Zheng X, Huang Y, Shan H, Huang J (2020) Successful use of methylprednisolone for treating severe COVID-19. J Allergy Clin Immunol 146:325–327. https://doi.org/10.1016/j.jaci.2020.05.021
Emori T, Kasahara M, Sugahara S, Hashimoto M, Ito H, Narumiya S, Higashi Y, Fujii Y (2020) Role of JAK-STAT signaling in the pathogenic behavior of fibroblast-like synoviocytes in rheumatoid arthritis: effect of the novel JAK inhibitor peficitinib. Eur J Pharmacol 882:173238. https://doi.org/10.1016/j.ejphar.2020.173238
Guo X, Li W, Li Q, Chen Y, Zhao G, Peng Y, Zheng J (2019) Tofacitinib is a mechanism-based inactivator of cytochrome P450 3A4. Chem Res Toxicol 32:1791–1800. https://doi.org/10.1021/acs.chemrestox.9b00141
Elli EM, Barate C, Mendicino F, Palandri F, Palumbo GA (2019) Mechanisms underlying the anti-inflammatory and immunosuppressive activity of ruxolitinib. Front Oncol 9:1186. https://doi.org/10.3389/fonc.2019.01186
Oscanoa TJ, Romero-Ortuno R, Carvajal A, Savarino A (2020) A pharmacological perspective of chloroquine in SARS-CoV-2 infection. Int J Antimicrob Agents 56:106078. https://doi.org/10.1016/j.ijantimicag.2020.106078
Graves PR, Kwiek JJ, Fadden P, Ray R, Hardeman K, Coley AM, Foley M, Haystead TA (2002) Discovery of novel targets of quinoline drugs in the human purine binding proteome. Mol Pharmacol 62:1364–1372. https://doi.org/10.1124/mol.62.6.1364
O'Hanlon R, Leyva-Grado VH, Sourisseau M, Evans MJ, Shaw ML (2019) An influenza virus entry inhibitor targets class II PI3 kinase and synergizes with oseltamivir. ACS Infect Dis 5:1779–1793. https://doi.org/10.1021/acsinfecdis.9b00230
Ono H (2019) Hypothermic action of oseltamivir not dependent on its anti-influenza virus action. Yakugaku Zasshi 139:767–781. https://doi.org/10.1248/yakushi.18-00191
De Clercq E (2002) New developments in anti-HIV chemotherapy. Biochim Biophys Acta 1587:258–275. https://doi.org/10.1016/s0925-4439(02)00089-3
Paskas S, Mazzon E, Basile MS, Cavalli E, Al-Abed Y, He M, Rakocevic S, Nicoletti F, Mijatovic S, Maksimovic-Ivanic D (2019) Lopinavir-NO, a nitric oxide-releasing HIV protease inhibitor, suppresses the growth of melanoma cells in vitro and in vivo. Invest New Drugs 37:1014–1028. https://doi.org/10.1007/s10637-019-00733-3
Mesa RA (2010) Ruxolitinib, a selective JAK1 and JAK2 inhibitor for the treatment of myeloproliferative neoplasms and psoriasis. IDrugs 13:394–403
Fu C, Sikandar A, Donner J, Zaburannyi N, Herrmann J, Reck M, Wagner-Dobler I, Koehnke J, Muller R (2017) The natural product carolacton inhibits folate-dependent C1 metabolism by targeting FolD/MTHFD. Nat Commun 8:1529. https://doi.org/10.1038/s41467-017-01671-5
Chen IS, Kubo Y (2018) Ivermectin and its target molecules: shared and unique modulation mechanisms of ion channels and receptors by ivermectin. J Physiol 596:1833–1845. https://doi.org/10.1113/JP275236
Li D, Zhang Y, Zhao RN, Fan S, Han JG (2014) Investigation on the mechanism for the binding and drug resistance of wild type and mutations of G86 residue in HIV-1 protease complexed with darunavir by molecular dynamic simulation and free energy calculation. J Mol Model 20:2122. https://doi.org/10.1007/s00894-014-2122-y
McEvoy GK (2003) AHFS drug information 2003. American Society of Health-System Pharmacists, Bethesda
Kumar V, Dhanjal JK, Bhargava P, Kaul A, Wang J, Zhang H, Kaul SC, Wadhwa R, Sundar D (2020) Withanone and withaferin-A are predicted to interact with transmembrane protease serine 2 (TMPRSS2) and block entry of SARS-CoV-2 into cells. J Biomol Struct Dyn 1–3. https://doi.org/10.1080/07391102.2020.1775704
Pardanani A, Harrison C, Cortes JE, Cervantes F, Mesa RA, Milligan D, Masszi T, Mishchenko E, Jourdan E, Vannucchi AM, Drummond MW, Jurgutis M, Kuliczkowski K, Gheorghita E, Passamonti F, Neumann F, Patki A, Gao G, Tefferi A (2015) Safety and efficacy of fedratinib in patients with primary or secondary myelofibrosis: a randomized clinical trial. JAMA Oncol 1:643–651. https://doi.org/10.1001/jamaoncol.2015.1590
Ng KE (2019) Xofluza (baloxavir marboxil) for the treatment of acute uncomplicated influenza. Pharm Ther 44:9–11
Zhang JN, Wang WJ, Peng B, Peng W, Zhang YS, Wang YL, Wan Y, Chang J, Mao L, Miao XP, Li YN, Zhou YF, Hu B (2020) Potential of arbidol for post-exposure prophylaxis of COVID-19 transmission: a preliminary report of a retrospective cohort study. Curr Med Sci 40:480–485. https://doi.org/10.1007/s11596-020-2203-3
Huet T, Beaussier H, Voisin O, Jouveshomme S, Dauriat G, Lazareth I, Sacco E, Naccache JM, Bézie Y, Laplanche S, Le Berre A, Le Pavec J, Salmeron S, Emmerich J, Mourad JJ, Chatellier G, Hayem G (2020) Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol 2(7):e393–e400. https://doi.org/10.1016/S2665-9913(20)30164-8
Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R (2003) Effects of chloroquine on viral infections: an old drug against today's diseases? Lancet Infect Dis 3:722–727. https://doi.org/10.1016/s1473-3099(03)00806-5
Yan Y, Zou Z, Sun Y, Li X, Xu KF, Wei Y, Jin N, Jiang C (2013) Anti-malaria drug chloroquine is highly effective in treating avian influenza A H5N1 virus infection in an animal model. Cell Res 23:300–302. https://doi.org/10.1038/cr.2012.165
Gao J, Tian Z, Yang X (2020) Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends 14:72–73. https://doi.org/10.5582/bst.2020.01047
Biot C, Daher W, Chavain N, Fandeur T, Khalife J, Dive D, De Clercq E (2006) Design and synthesis of hydroxyferroquine derivatives with antimalarial and antiviral activities. J Med Chem 49:2845–2849. https://doi.org/10.1021/jm0601856
Marmor MF, Kellner U, Lai TY, Melles RB, Mieler WF, A, American Academy of O (2016) Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology 123:1386–1394. https://doi.org/10.1016/j.ophtha.2016.01.058
Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, Tissot Dupont H, Honore S, Colson P, Chabriere E, La Scola B, Rolain JM, Brouqui P, Raoult D (2020) Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. https://doi.org/10.1016/j.ijantimicag.2020.105949
Zhou D, Dai SM, Tong Q (2020) COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J Antimicrob Chemother 75:1667–1670. https://doi.org/10.1093/jac/dkaa114
Oestereich L, Ludtke A, Wurr S, Rieger T, Munoz-Fontela C, Gunther S (2014) Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antiviral Res 105:17–21. https://doi.org/10.1016/j.antiviral.2014.02.014
Coomes EA, Haghbayan H (2020) Favipiravir, an antiviral for COVID-19? J Antimicrob Chemother 75:2013–2014. https://doi.org/10.1093/jac/dkaa171
Du YX, Chen XP (2020) Favipiravir: pharmacokinetics and concerns about clinical trials for 2019-nCoV infection. Clin Pharmacol Ther 108:242–247. https://doi.org/10.1002/cpt.1844
de Wit E, Feldmann F, Cronin J, Jordan R, Okumura A, Thomas T, Scott D, Cihlar T, Feldmann H (2020) Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci USA 117:6771–6776. https://doi.org/10.1073/pnas.1922083117
Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G (2020) Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 30:269–271. https://doi.org/10.1038/s41422-020-0282-0
Shannon A, Le NT, Selisko B, Eydoux C, Alvarez K, Guillemot JC, Decroly E, Peersen O, Ferron F, Canard B (2020) Remdesivir and SARS-CoV-2: structural requirements at both nsp12 RdRp and nsp14 exonuclease active-sites. Antiviral Res 178:104793. https://doi.org/10.1016/j.antiviral.2020.104793
Yehudai D, Liyanage SU, Hurren R, Rizoska B, Albertella M, Gronda M, Jeyaraju DV, Wang X, Barghout SH, MacLean N, Siriwardena TP, Jitkova Y, Targett-Adams P, Schimmer AD (2019) The thymidine dideoxynucleoside analog, alovudine, inhibits the mitochondrial DNA polymerase gamma, impairs oxidative phosphorylation and promotes monocytic differentiation in acute myeloid leukemia. Haematologica 104:963–972. https://doi.org/10.3324/haematol.2018.195172
Sarzi-Puttini P, Giorgi V, Sirotti S, Marotto D, Ardizzone S, Rizzardini G, Antinori S, Galli M (2020) COVID-19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clin Exp Rheumatol 38:337–342
Richardson P, Griffin I, Tucker C, Smith D, Oechsle O, Phelan A, Stebbing J (2020) Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet 395:e30–e31. https://doi.org/10.1016/S0140-6736(20)30304-4
Stebbing J, Phelan A, Griffin I, Tucker C, Oechsle O, Smith D, Richardson P (2020) COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis 20:400–402. https://doi.org/10.1016/S1473-3099(20)30132-8
Fridman JS, Scherle PA, Collins R, Burn TC, Li Y, Li J, Covington MB, Thomas B, Collier P, Favata MF, Wen X, Shi J, McGee R, Haley PJ, Shepard S, Rodgers JD, Yeleswaram S, Hollis G, Newton RC, Metcalf B, Friedman SM, Vaddi K (2010) Selective inhibition of JAK1 and JAK2 is efficacious in rodent models of arthritis: preclinical characterization of INCB028050. J Immunol 184:5298–5307. https://doi.org/10.4049/jimmunol.0902819
Angelini J, Talotta R, Roncato R, Fornasier G, Barbiero G, Dal Cin L, Brancati S, Scaglione F (2020) JAK-inhibitors for the treatment of rheumatoid arthritis: a focus on the present and an outlook on the future. Biomolecules 10:1002. https://doi.org/10.3390/biom10071002
Best JH, Vlad SC, Tominna L, Abbass I (2020) Real-world persistence with tocilizumab compared to other subcutaneous biologic disease-modifying antirheumatic drugs among patients with rheumatoid arthritis switching from another biologic. Rheumatol Ther 7:345–355. https://doi.org/10.1007/s40744-020-00201-y
Gray KA, Daugherty LC, Gordon SM, Seal RL, Wright MW, Bruford EA (2013) Genenames.org: the HGNC resources in 2013. Nucleic Acids Res 41:D545–D552. https://doi.org/10.1093/nar/gks1066
Mariano VJ, Frishman WH (2018) Tocilizumab in giant cell arteritis. Cardiol Rev 26:321–330. https://doi.org/10.1097/CRD.0000000000000204
Fu B, Xu X, Wei H (2020) Why tocilizumab could be an effective treatment for severe COVID-19? J Transl Med 18:164. https://doi.org/10.1186/s12967-020-02339-3
Lythgoe MP, Middleton P (2020) Ongoing clinical trials for the management of the COVID-19 pandemic. Trends Pharmacol Sci 41:363–382. https://doi.org/10.1016/j.tips.2020.03.006
FDA Approved Drug Products: Kaletra (lopinavir/ritonavir) for oral use: https://go.drugbank.com/drugs/DB01601
Cao J, Forrest JC, Zhang X (2015) A screen of the NIH clinical collection small molecule library identifies potential anti-coronavirus drugs. Antiviral Res 114:1–10. https://doi.org/10.1016/j.antiviral.2014.11.010
Hung IF, Lung KC, Tso EY, Liu R, Chung TW, Chu MY, Ng YY, Lo J, Chan J, Tam AR, Shum HP, Chan V, Wu AK, Sin KM, Leung WS, Law WL, Lung DC, Sin S, Yeung P, Yip CC, Zhang RR, Fung AY, Yan EY, Leung KH, Ip JD, Chu AW, Chan WM, Ng AC, Lee R, Fung K, Yeung A, Wu TC, Chan JW, Yan WW, Chan WM, Chan JF, Lie AK, Tsang OT, Cheng VC, Que TL, Lau CS, Chan KH, To KK, Yuen KY (2020) Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet 395:1695–1704. https://doi.org/10.1016/S0140-6736(20)31042-4
Uno Y (2020) Camostat mesilate therapy for COVID-19. Intern Emerg Med 29:1–2. https://doi.org/10.1007/s11739-020-02345-9
Coote K, Atherton-Watson HC, Sugar R, Young A, MacKenzie-Beevor A, Gosling M, Bhalay G, Bloomfield G, Dunstan A, Bridges RJ, Sabater JR, Abraham WM, Tully D, Pacoma R, Schumacher A, Harris J, Danahay H (2009) Camostat attenuates airway epithelial sodium channel function in vivo through the inhibition of a channel-activating protease. J Pharmacol Exp Ther 329:764–774. https://doi.org/10.1124/jpet.108.148155
Yamauchi J, Takeda K, Shibuya K, Sunamura M, Matsuno S (2001) Continuous regional application of protease inhibitor in the treatment of acute pancreatitis. An experimental study using closed duodenal obstruction model in dogs. Pancreatology 1:662–667. https://doi.org/10.1159/000055878
Andersen PI, Krpina K, Ianevski A, Shtaida N, Jo E, Yang J, Koit S, Tenson T, Hukkanen V, Anthonsen MW, Bjoras M, Evander M, Windisch MP, Zusinaite E, Kainov DE (2019) Novel antiviral activities of obatoclax, emetine, niclosamide, brequinar, and homoharringtonine. Viruses 11:964. https://doi.org/10.3390/v11100964
Choy KT, Wong AY, Kaewpreedee P, Sia SF, Chen D, Hui KPY, Chu DKW, Chan MCW, Cheung PP, Huang X, Peiris M, Yen HL (2020) Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral Res 178:104786. https://doi.org/10.1016/j.antiviral.2020.104786
Zhu Z, Chakraborti S, He Y, Roberts A, Sheahan T, Xiao X, Hensley LE, Prabakaran P, Rockx B, Sidorov IA, Corti D, Vogel L, Feng Y, Kim JO, Wang LF, Baric R, Lanzavecchia A, Curtis KM, Nabel GJ, Subbarao K, Jiang S, Dimitrov DS (2007) Potent cross-reactive neutralization of SARS coronavirus isolates by human monoclonal antibodies. Proc Natl Acad Sci USA 104:12123–12128. https://doi.org/10.1073/pnas.0701000104
Rockx B, Corti D, Donaldson E, Sheahan T, Stadler K, Lanzavecchia A, Baric R (2008) Structural basis for potent cross-neutralizing human monoclonal antibody protection against lethal human and zoonotic severe acute respiratory syndrome coronavirus challenge. J Virol 82:3220–3235. https://doi.org/10.1128/JVI.02377-07
Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL (2020) A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579:270–273. https://doi.org/10.1038/s41586-020-2012-7
Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, Zhang Q, Shi X, Wang Q, Zhang L, Wang X (2020) Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 581:215–220. https://doi.org/10.1038/s41586-020-2180-5
Du L, He Y, Zhou Y, Liu S, Zheng BJ, Jiang S (2009) The spike protein of SARS-CoV–a target for vaccine and therapeutic development. Nat Rev Microbiol 7:226–236. https://doi.org/10.1038/nrmicro2090
Dazzi F, Horwood NJ (2007) Potential of mesenchymal stem cell therapy. Curr Opin Oncol 19:650–655. https://doi.org/10.1097/CCO.0b013e3282f0e116
Moreira A, Kahlenberg S, Hornsby P (2017) Therapeutic potential of mesenchymal stem cells for diabetes. J Mol Endocrinol 59:R109–R120. https://doi.org/10.1530/JME-17-0117
Golchin A, Farahany TZ, Khojasteh A, Soleimanifar F, Ardeshirylajimi A (2019) The clinical trials of mesenchymal stem cell therapy in skin diseases: an update and concise review. Curr Stem Cell Res Ther 14:22–33. https://doi.org/10.2174/1574888X13666180913123424
Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, Shan G, Meng F, Du D, Wang S, Fan J, Wang W, Deng L, Shi H, Li H, Hu Z, Zhang F, Gao J, Liu H, Li X, Zhao Y, Yin K, He X, Gao Z, Wang Y, Yang B, Jin R, Stambler I, Lim LW, Su H, Moskalev A, Cano A, Chakrabarti S, Min KJ, Ellison-Hughes G, Caruso C, Jin K, Zhao RC (2020) Transplantation of ACE2(-) mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis 11:216–228. https://doi.org/10.14336/AD.2020.0228
Liang B, Chen J, Li T, Wu H, Yang W, Li Y, Li J, Yu C, Nie F, Ma Z, Yang M, Xiao M, Nie P, Gao Y, Qian C, Hu M (2020) Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells: a case report. Medicine (Baltimore) 99:e21429. https://doi.org/10.1097/MD.0000000000021429
Funding
MKK is the recipient of the TARE fellowship (Grant # TAR/2018/001054) from the Science and Engineering Research Board (SERB), Department of Science and Technology, Government of India.
Author information
Authors and Affiliations
Contributions
MKK and AH conceived and guided the research. MKK, AKS, PK, AK, GT, & AT carried out the exhaustive search on the data. MKK, AH, AKS, PK, AK, GT, AT, KM, and PCM analyzed and interpreted the data. MKK wrote the manuscripts. All the authors read, critically evaluated, gave their feedback, and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no potential conflicts of interest and also no competing financial interest associated with this manuscript.
Ethical approval
The data included in the study was collected form published studied on COVID-19. The study does not involve any human subjects, samples or cell lines.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kumar, P., Sah, A.K., Tripathi, G. et al. Role of ACE2 receptor and the landscape of treatment options from convalescent plasma therapy to the drug repurposing in COVID-19. Mol Cell Biochem 476, 553–574 (2021). https://doi.org/10.1007/s11010-020-03924-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03924-2