Abstract
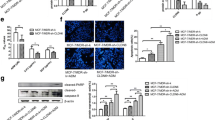
Claudin-6 (CLDN6), a critical tight junction protein acting as a tumor suppressor in breast cancer, is also considered to be a stem cell marker. Triple-negative breast cancer (TNBC) is a subtype of claudin-low and stem cell-like breast cancer which is chemoresistant to multiple anti-cancer drugs. The aim of our study was to determine whether CLDN6 plays a role in chemoresistance of TNBC. We found that overexpression of CLDN6 in TNBC cell line MDAMB231 significantly inhibited cell growth, migration, and invasion. The expression of CLDN6 increased the IC50 of adriamycin (ADM) and promoted the clonogenic survival. CLDN6 inhibited ADM-induced apoptosis and senescence in MDAMB231 cells. However, P-gp, a resistance-related protein highly associated with chemoresistance, was downregulated by CLDN6 overexpression in MDAMB231 cells. Epithelial mesenchymal transition (EMT) marker E-cadherin was increased, and vimentin was decreased by CLDN6. In addition, stem cell markers OCT4, SOX2, and Nanog were dramatically increased. CLDN6 colocalized and interacted with AF-6. Overexpression of CLDN6 increased the expression of afadin (AF-6) and hampered the activation of ERK signaling. PMA, a specific ERK activator, reversed the expression of EMT and stem cell markers, and decreased chemoresistance of MDAMB231 cells to ADM with a decreased IC50 and an increased apoptosis resulting from CLDN6. Together, we conclude that CLDN6 enhances the chemoresistance to ADM via activating the AF-6/ERK signaling pathway and up-regulating cancer stem cell characters in MDAMB231 cells.






Similar content being viewed by others
References
Bosch A, Eroles P, Zaragoza R, Vina JR, Lluch A (2010) Triple-negative breast cancer: molecular features, pathogenesis, treatment and current lines of research. Cancer Treat Rev 36:206–215. https://doi.org/10.1016/j.ctrv.2009.12.002
Carey LA (2011) Directed therapy of subtypes of triple-negative breast cancer. Oncologist 16(Suppl 1):71–78
Abdullah LN, Chow EK (2013) Mechanisms of chemoresistance in cancer stem cells. Clin Transl Med 2:3. https://doi.org/10.1186/2001-1326-2-3
Xu X, Jin H, Liu Y, Liu L, Wu Q, Guo Y, Yu L, Liu Z, Zhang T, Zhang X, Dong X, Quan C (2012) The expression patterns and correlations of claudin-6, methy-CpG binding protein 2, DNA methyltransferase 1, histone deacetylase 1, acetyl-histone H3 and acetyl-histone H4 and their clinicopathological significance in breast invasive ductal carcinomas. Diagn Pathol 7:33. https://doi.org/10.1186/1746-1596-7-33
Zavala-Zendejas VE, Torres-Martinez AC, Salas-Morales B, Fortoul TI, Montano LF, Rendon-Huerta EP (2011) Claudin-6, 7, or 9 overexpression in the human gastric adenocarcinoma cell line AGS increases its invasiveness, migration, and proliferation rate. Cancer Investig 29:1–11. https://doi.org/10.3109/07357907.2010.512594
Osanai M, Murata M, Chiba H, Kojima T, Sawada N (2007) Epigenetic silencing of claudin-6 promotes anchorage-independent growth of breast carcinoma cells. Cancer Sci 98:1557–1562. https://doi.org/10.1111/j.1349-7006.2007.00569.x
Wu Q, Liu Y, Ren Y, Xu X, Yu L, Li Y, Quan C (2010) Tight junction protein, claudin-6, downregulates the malignant phenotype of breast carcinoma. Eur J Cancer Prev 19:186 –94. https://doi.org/10.1097/CEJ.0b013e328337210e
Wang L, Xue Y, Shen Y, Li W, Cheng Y, Yan X, Shi W, Wang J, Gong Z, Yang G, Guo C, Zhou Y, Wang X, Zhou Q, Zeng F (2012) Claudin 6: a novel surface marker for characterizing mouse pluripotent stem cells. Cell Res 22:1082–1085. https://doi.org/10.1038/cr.2012.77
Ben-David U, Nudel N, Benvenisty N (2013) Immunologic and chemical targeting of the tight-junction protein Claudin-6 eliminates tumorigenic human pluripotent stem cells. Nat Commun 4:1992. https://doi.org/10.1038/ncomms2992
Chiou SH, Wang ML, Chou YT, Chen CJ, Hong CF, Hsieh WJ, Chang HT, Chen YS, Lin TW, Hsu HS, Wu CW (2010) Coexpression of Oct4 and Nanog enhances malignancy in lung adenocarcinoma by inducing cancer stem cell-like properties and epithelial-mesenchymal transdifferentiation. Cancer Res 70:10433–10444. https://doi.org/10.1158/0008-5472.CAN-10-2638
Shang X, Lin X, Manorek G, Howell SB (2013) Claudin-3 and claudin-4 regulate sensitivity to cisplatin by controlling expression of the copper and cisplatin influx transporter CTR1. Mol Pharmacol 83:85–94. https://doi.org/10.1124/mol.112.079798
Casagrande F, Cocco E, Bellone S, Richter CE, Bellone M, Todeschini P, Siegel E, Varughese J, Arin-Silasi D, Azodi M, Rutherford TJ, Pecorelli S, Schwartz PE, Santin AD (2011) Eradication of chemotherapy-resistant CD44 + human ovarian cancer stem cells in mice by intraperitoneal administration of Clostridium perfringens enterotoxin. Cancer 117:5519–5528. https://doi.org/10.1002/cncr.26215
Lin X, Shang X, Manorek G, Howell SB (2013) Regulation of the epithelial-mesenchymal transition by claudin-3 and claudin-4. PLoS ONE 8:e67496. https://doi.org/10.1371/journal.pone.0067496
Kim CJ, Lee JW, Choi JJ, Choi HY, Park YA, Jeon HK, Sung CO, Song SY, Lee YY, Choi CH, Kim TJ, Lee JH, Kim BG, Bae DS (2011) High claudin-7 expression is associated with a poor response to platinum-based chemotherapy in epithelial ovarian carcinoma. Eur J Cancer 47:918–925. https://doi.org/10.1016/j.ejca.2010.11.007
Thuma F, Zoller M (2013) EpCAM-associated claudin-7 supports lymphatic spread and drug resistance in rat pancreatic cancer. Int J Cancer 133:855 – 66. https://doi.org/10.1002/ijc.28085
Nomura Y (1996) [Adriamycin–breast cancer]. Gan To Kagaku Ryoho 23:1911–1915
Wong HL, Bendayan R, Rauth AM, Xue HY, Babakhanian K, Wu XY (2006) A mechanistic study of enhanced doxorubicin uptake and retention in multidrug resistant breast cancer cells using a polymer-lipid hybrid nanoparticle system. J Pharmacol Exp Ther 317:1372–1381. https://doi.org/10.1124/jpet.106.101154
Shen F, Chu S, Bence AK, Bailey B, Xue X, Erickson PA, Montrose MH, Beck WT, Erickson LC (2008) Quantitation of doxorubicin uptake, efflux, and modulation of multidrug resistance (MDR) in MDR human cancer cells. J Pharmacol Exp Ther 324:95–102. https://doi.org/10.1124/jpet.107.127704
Ren Y, Wu Q, Liu Y, Xu X, Quan C (2013) Gene silencing of claudin6 enhances cell proliferation and migration accompanied with increased MMP2 activity via p38 MAPK signaling pathway in human breast epithelium cell line HBL100. Mol Med Rep 8:1505–1510. https://doi.org/10.3892/mmr.2013.1675
Arabzadeh A, Troy TC, Turksen K (2006) Role of the Cldn6 cytoplasmic tail domain in membrane targeting and epidermal differentiation in vivo. Mol Cell Biol 26:5876–5887. https://doi.org/10.1128/MCB.02342-05
Turksen K, Troy TC (2001) Claudin-6: a novel tight junction molecule is developmentally regulated in mouse embryonic epithelium. Dev Dyn 222:292–300. https://doi.org/10.1002/dvdy.1174
Quan C, Lu SJ (2003) Identification of genes preferentially expressed in mammary epithelial cells of Copenhagen rat using subtractive hybridization and microarrays. Carcinogenesis 24:1593–1599. https://doi.org/10.1093/carcin/bgg129
Guo Y, Xu X, Liu Z, Zhang T, Zhang X, Wang L, Wang M, Liu Y, Lu Y, Liu Y, Quan C (2012) Apoptosis signal-regulating kinase 1 is associated with the effect of claudin-6 in breast cancer. Diagn Pathol 7:111. https://doi.org/10.1186/1746-1596-7-111
Guo Y, Lin D, Zhang M, Zhang X, Li Y, Yang R, Lu Y, Jin X, Yang M, Wang M, Zhao S, Quan C (2016) CLDN6-induced apoptosis via regulating ASK1-p38/JNK signaling in breast cancer MCF-7 cells. Int J Oncol 48:2435–2444. https://doi.org/10.3892/ijo.2016.3469
Oba T, Izumi H, Ito KI (2016) ABCB1 and ABCC11 confer resistance to eribulin in breast cancer cell lines. Oncotarget 7:70011–70027. https://doi.org/10.18632/oncotarget.11727
Hansen SN, Westergaard D, Thomsen MB, Vistesen M, Do KN, Fogh L, Belling KC, Wang J, Yang H, Gupta R, Ditzel HJ, Moreira J, Brunner N, Stenvang J, Schrohl AS (2015) Acquisition of docetaxel resistance in breast cancer cells reveals upregulation of ABCB1 expression as a key mediator of resistance accompanied by discrete upregulation of other specific genes and pathways. Tumour Biol 36:4327–4338. https://doi.org/10.1007/s13277-015-3072-4
Kwon MJ (2013) Emerging roles of claudins in human cancer. Int J Mol Sci 14:18148–18180. https://doi.org/10.3390/ijms140918148
Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, Wu CC, LeBleu VS, Kalluri R (2015) Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 527:525–530. https://doi.org/10.1038/nature16064
Fischer KR, Durrans A, Lee S, Sheng J, Li F, Wong ST, Choi H, El Rayes T, Ryu S, Troeger J, Schwabe RF, Vahdat LT, Altorki NK, Mittal V, Gao D (2015) Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 527:472–476. https://doi.org/10.1038/nature15748
Rosano L, Cianfrocca R, Spinella F, Di Castro V, Nicotra MR, Lucidi A, Ferrandina G, Natali PG, Bagnato A (2011) Acquisition of chemoresistance and EMT phenotype is linked with activation of the endothelin A receptor pathway in ovarian carcinoma cells. Clin Cancer Res 17:2350–2360. https://doi.org/10.1158/1078-0432.CCR-10-2325
Ren J, Chen Y, Song H, Chen L, Wang R (2013) Inhibition of ZEB1 reverses EMT and chemoresistance in docetaxel-resistant human lung adenocarcinoma cell line. J Cell Biochem 114:1395–403. https://doi.org/10.1002/jcb.24481
Hamada S, Masamune A, Takikawa T, Suzuki N, Kikuta K, Hirota M, Hamada H, Kobune M, Satoh K, Shimosegawa T (2012) Pancreatic stellate cells enhance stem cell-like phenotypes in pancreatic cancer cells. Biochem Biophys Res Commun 421:349–354. https://doi.org/10.1016/j.bbrc.2012.04.014
Steg AD, Bevis KS, Katre AA, Ziebarth A, Dobbin ZC, Alvarez RD, Zhang K, Conner M, Landen CN (2012) Stem cell pathways contribute to clinical chemoresistance in ovarian cancer. Clin Cancer Res 18:869–881. https://doi.org/10.1158/1078-0432.CCR-11-2188
Tannock IF (2015) Cancer: resistance through repopulation. Nature 517:152–153. https://doi.org/10.1038/nature14075
Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S (1999) Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol 147:1351–1363
Zhadanov AB, Provance DW Jr, Speer CA, Coffin JD, Goss D, Blixt JA, Reichert CM, Mercer JA (1999) Absence of the tight junctional protein AF-6 disrupts epithelial cell-cell junctions and cell polarity during mouse development. Curr Biol 9:880–888
Yamamoto T, Harada N, Kano K, Taya S, Canaani E, Matsuura Y, Mizoguchi A, Ide C, Kaibuchi K (1997) The Ras target AF-6 interacts with ZO-1 and serves as a peripheral component of tight junctions in epithelial cells. J Cell Biol 139:785–795
Yamamoto T, Mori T, Sawada M, Matsushima H, Ito F, Akiyama M, Kitawaki J (2015) Loss of AF-6/afadin induces cell invasion, suppresses the formation of glandular structures and might be a predictive marker of resistance to chemotherapy in endometrial cancer. BMC Cancer 15:275. https://doi.org/10.1186/s12885-015-1286-x
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (Code: 81172499) and Science and Technology Development Plan of the Office of Science and Technology Project in Jilin Province (Code: 20140414036GH).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, M., Li, Y., Ruan, Y. et al. CLDN6 enhances chemoresistance to ADM via AF-6/ERKs pathway in TNBC cell line MDAMB231. Mol Cell Biochem 443, 169–180 (2018). https://doi.org/10.1007/s11010-017-3221-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-017-3221-8