Abstract
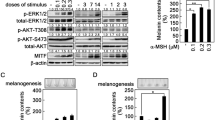
Melanin production within melanocytes is regulated, among others, by estradiol, whose effects on melanogenesis are still not completely elucidated. Here we show that although 10−7 M 17β-estradiol (E2) increased tyrosinase mRNA levels in B16-F10 malignant melanocytes, there was a transient decrease and abolishment of the temporal variation of melanin content. Both parameters were much higher in the malignant than in normal Melan-a cells. Considering that silencing clock machinery in human melanocytes increases melanogenesis, we investigated clock gene expression in those cell lines. Except for Melan-a Bmal1 and B16-F10 Per2 expression of control cells, Per1, Per2, and Bmal1 expression increased independently of cell type or E2 treatment after 24 h. However, melanoma cells showed a marked increase in Per1 and Bma11 expression in response to E2 at the same time points, what may rule out E2 as a synchronizer agent since the expression of those genes were not in antiphase. Next, we investigated the expression of Xpa, a clock-controlled gene, which in Melan-a cells, peaked at 18 h, and E2 treatment shifted this peak to 24 h, whereas B16-F10 Xpa expression peaked at 24 h in both control and E2 group, and it was higher compared to Melan-a cells in both groups. Therefore, malignant and normal melanocytes display profound differences on core elements of the local clock, and how they respond to E2, what is most probably determinant of the differences seen on melanin synthesis and Tyrosinase and Xpa expression. Understanding these processes at the molecular level could bring new strategies to treat melanoma.




Similar content being viewed by others
References
Sandu C, Dumas M, Malan A, Sambakhe D, Marteau C, Nizard C, Schnebert S, Perrier E, Challet E, Pevet P, Felder-Schmittbuhl MP (2012) Human skin keratinocytes, melanocytes, and fibroblasts contain distinct circadian clock machineries. Cell Mol Life Sci 69:3329–3339. doi:10.1007/s00018-012-1026-1
Orlow SJ (1995) Melanosomes are specialized members of the lysosomal lineage of organelles. J Invest Dermatol 105:3–7
Marks MS, Seabra MC (2001) The melanosome: membrane dynamics in black and white. Nat Rev Mol Cell Biol 2:738–748. doi:10.1038/35096009
Sklar LR, Almutawa F, Lim HW, Hamzavi I (2013) Effects of ultraviolet radiation, visible light, and infrared radiation on erythema and pigmentation: a review. Photochem Photobiol Sci 12:54–64. doi:10.1039/c2pp25152c
Lin JY, Fisher DE (2007) Melanocyte biology and skin pigmentation. Nature 445:843–850. doi:10.1038/nature05660
Solano F (2014) Melanins: skin pigments and much more—types, structural models, biological functions, and formation routes. N J Sci 2014:28. doi:10.1155/2014/498276
Slominski A, Tobin DJ, Shibahara S, Wortsman J (2004) Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev 84:1155–1228. doi:10.1152/physrev.00044.2003
McGregor JM, Hawk JLM (1999) Acute effects of ultraviolet radiation on the skin. In: Freedberg IM, Eisen AZ, Wolff K (eds) Fitzpatrick’s dermatology in general medicine, 5th edn. McGraw-Hill, New York, pp 1555–1561
Dupont E, Gomez J, Bilodeau D (2013) Beyond UV radiation: a skin under challenge. Int J Cosmet Sci 35:224–232. doi:10.1111/ics.12036
Premi S, Wallisch S, Mano CM, Weiner AB, Bacchiocchi A, Wakamatsu K, Bechara EJ, Halaban R, Douki T, Brash DE (2015) Photochemistry. Chemiexcitation of melanin derivatives induces DNA photoproducts long after UV exposure. Science 347:842–847. doi:10.1126/science.1256022
Eller MS, Ostrom K, Gilchrest BA (1996) DNA damage enhances melanogenesis. Proc Natl Acad Sci USA 93:1087–1092
Brenner M, Hearing VJ (2008) The protective role of melanin against UV damage in human skin. Photochem Photobiol 84:539–549. doi:10.1111/j.1751-1097.2007.00226
Smith QT, Allison DJ (1966) Changes of collagen content in skin, femur and uterus of 17-beta-estradiol benzoate-treated rats. Endocrinology 79:486–492. doi:10.1210/endo-79-3-486
Henneman DH (1968) Effect of estrogen on in vivo and in vitro collagen biosynthesis and maturation in old and young female guinea pigs. Endocrinology 83:678–690. doi:10.1210/endo-83-4-678
Sobel H, Lee KD, Hewlett MJ (1965) Effect of estrogen on acid glycosaminoglycans in skin of mice. Biochim Biophys Acta 101:225–229
Bullough HF (1947) Epidermal thickness following oestrone injections in the mouse. Nature 159:101–102. doi:10.1038/159101a0
Maeda K, Naganuma M, Fukuda M, Matsunaga J, Tomita Y (1996) Effect of pituitary and ovarian hormones on human melanocytes in vitro. Pigment Cell Res 9:204–212
Jee SH, Lee SY, Chiu HC, Chang CC, Chen TJ (1994) Effects of estrogen and estrogen receptor in normal human melanocytes. Biochem Biophys Res Commun 199:1407–1412. doi:10.1006/bbrc.1994.1387
McLeod SD, Ranson M, Mason RS (1994) Effects of estrogens on human melanocytes in vitro. J Steroid Biochem Mol Biol 49:9–14
Lupulescu A (1981) Hormonal regulation of epidermal tumor development. J Invest Dermatol 77:186–195
Yamaguchi S, Mitsui S, Yan L, Yagita K, Miyake S, Okamura H (2000) Role of DBP in the circadian oscillatory mechanism. Mol Cell Biol 20:4773–4781
Buhr ED, Takahashi JS (2013) Molecular components of the mammalian circadian clock. Handb Exp Pharmacol. doi:10.1007/978-3-642-25950-0_1
Partch CL, Green CB, Takahashi JS (2014) Molecular architecture of the mammalian circadian clock. Trends Cell Biol 24:90–99. doi:10.1016/j.tcb.2013.07.002
Luber AJ, Ensanyat SH, Zeichner JA (2014) Therapeutic implications of the circadian clock on skin function. J Drugs Dermatol 13:130–144
Gaddameedhi S, Selby CP, Kaufmann WK, Smart RC, Sancar A (2011) Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci USA 108:18790–18795. doi:10.1073/pnas.1115249108
Matsunaga N, Itcho K, Hamamura K, Ikeda E, Ikeyama H, Furuichi Y, Watanabe M, Koyanagi S, Ohdo S (2014) 24-hour rhythm of aquaporin-3 function in the epidermis is regulated by molecular clocks. J Invest Dermatol 134:1636–1644. doi:10.1038/jid.2014.13
Hardman JA, Tobin DJ, Haslam IS, Farjo N, Farjo B, Al-Nuaimi Y, Grimaldi B, Paus R (2015) The peripheral clock regulates human pigmentation. J Invest Dermatol 135:1053–1064. doi:10.1038/jid.2014.442
Sukumaran S, Almon RR, DuBois DC, Jusko WJ (2010) Circadian rhythms in gene expression: relationship to physiology, disease, drug disposition and drug action. Adv Drug Deliv Rev 62:904–917. doi:10.1016/j.addr.2010.05.009
Savvidis C, Koutsilieris M (2012) Circadian rhythm disruption in cancer biology. Mol Med 18:1249–1260. doi:10.2119/molmed.2012.00077
Kelleher FC, Rao Maguire A (2014) Circadian molecular clocks and cancer. Cancer Lett 342:9–18. doi:10.1016/j.canlet.2013.09.040
Lengyel Z, Lovig C, Kommedal S, Keszthelyi R, Szekeres G, Battyani Z, Csernus V, Nagy AD (2013) Altered expression patterns of clock gene mRNAs and clock proteins in human skin tumors. Tumour Biol 34:811–819. doi:10.1007/s13277-012-0611-0
de Assis LV, Moraes MN, da Silveira Cruz-Machado S, Castrucci AM (2016) The effect of white light on normal and malignant murine melanocytes: a link between opsins, clock genes, and melanogenesis. Biochim Biophys Acta 1863:1119–1133. doi:10.1016/j.bbamcr.2016.03.001
Tsuchiya Y, Akashi M, Nishida E (2003) Temperature compensation and temperature resetting of circadian rhythms in mammalian cultured fibroblasts. Genes Cells 8:713–720
Sporl F, Schellenberg K, Blatt T, Wenck H, Wittern KP, Schrader A, Kramer A (2011) A circadian clock in HaCaT keratinocytes. J Invest Dermatol 131:338–348. doi:10.1038/jid.2010.315
Sandu C, Liu T, Malan A, Challet E, Pevet P, Felder-Schmittbuhl MP (2015) Circadian clocks in rat skin and dermal fibroblasts: differential effects of aging, temperature and melatonin. Cell Mol Life Sci 72:2237–2248. doi:10.1007/s00018-014-1809-7
He PJ, Hirata M, Yamauchi N, Hattori MA (2007) Up-regulation of Per1 expression by estradiol and progesterone in the rat uterus. J Endocrinol 194:511–519. doi:10.1677/joe-07-0172
Nakamura TJ, Sellix MT, Menaker M, Block GD (2008) Estrogen directly modulates circadian rhythms of PER2 expression in the uterus. Am J Physiol Endocrinol Metab 295:E1025–E1031. doi:10.1152/ajpendo.90392.2008
Nakamura TJ, Moriya T, Inoue S, Shimazoe T, Watanabe S, Ebihara S, Shinohara K (2005) Estrogen differentially regulates expression of Per1 and Per2 genes between central and peripheral clocks and between reproductive and nonreproductive tissues in female rats. J Neurosci Res 82:622–630. doi:10.1002/jnr.20677
Bennett DC, Cooper PJ, Hart IR (1987) A line of non-tumorigenic mouse melanocytes, syngeneic with the B16 melanoma and requiring a tumor promoter for growth. Int J Cancer 39:414–418
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the \(2^{{ - \Delta \Delta c_{T} }}\) Method. Methods 25:402-408. doi: 10.1006/meth.2001.1262
Naeyaert JM, Eller M, Gordon PR, Park HY, Gilchrest BA (1991) Pigment content of cultured human melanocytes does not correlate with tyrosinase message level. Br J Dermatol 125:297–303
Iozumi K, Hoganson GE, Pennella R, Everett MA, Fuller BB (1993) Role of tyrosinase as the determinant of pigmentation in cultured human melanocytes. J Invest Dermatol 100:806–811
Haltaufderhyde KD, Oancea E (2014) Genome-wide transcriptome analysis of human epidermal melanocytes. Genomics 104:482–489. doi:10.1016/j.ygeno.2014.09.010
Iwata M, Corn T, Iwata S, Everett MA, Fuller BB (1990) The relationship between tyrosinase activity and skin color in human foreskins. J Invest Dermatol 95:9–15
Maeda K, Yokokawa Y, Hatao M, Naganuma M, Tomita Y (1997) Comparison of the melanogenesis in human black and light brown melanocytes. J Dermatol Sci 14:199–206
Murase D, Hachiya A, Fullenkamp R, Beck A, Moriwaki S, Hase T, Takema Y, Manga P (2016) Variation in Hsp70-1A expression contributes to skin color diversity. J Invest Dermatol. doi:10.1016/j.jid.2016.03.038
Filipski E, Subramanian P, Carriere J, Guettier C, Barbason H, Levi F (2009) Circadian disruption accelerates liver carcinogenesis in mice. Mutat Res 680:95–105. doi:10.1016/j.mrgentox.2009.10.002
Desotelle JA, Wilking MJ, Ahmad N (2012) The circadian control of skin and cutaneous photodamage. Photochem Photobiol 88:1037–1047. doi:10.1111/j.1751-1097.2012.01099.x
Balsalobre A, Damiola F, Schibler U (1998) A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell 93:929–937
Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H (2000) Resetting central and peripheral circadian oscillators in transgenic rats. Science 288:682–685
Hirota T, Okano T, Kokame K, Shirotani-Ikejima H, Miyata T, Fukada Y (2002) Glucose down-regulates Per1 and Per2 mRNA levels and induces circadian gene expression in cultured Rat-1 fibroblasts. J Biol Chem 277:44244–44251. doi:10.1074/jbc.M206233200
Albrecht U (2012) Timing to perfection: the biology of central and peripheral circadian clocks. Neuron 74:246–260. doi:10.1016/j.neuron.2012.04.006
Gery S, Virk RK, Chumakov K, Yu A, Koeffler HP (2007) The clock gene Per2 links the circadian system to the estrogen receptor. Oncogene 26:7916–79120. doi:10.1038/sj.onc.1210585
Sadoff L, Winkley J, Tyson S (1973) Is malignant melanoma an endocrine-dependent tumor? The possible adverse effect of estrogen. Oncology 27:244–257
Buzaid AC, Murren JR, Durivage HJ (1991) High-dose cisplatin with dacarbazine and tamoxifen in the treatment of metastatic melanoma. Cancer 68:1238–1241
McClay EF, Mastrangelo MJ, Berd D, Bellet RE (1992) Effective combination chemo/hormonal therapy for malignant melanoma: experience with three consecutive trials. Int J Cancer 50:553–556
Bhatia S, Tykodi SS, Thompson JA (2009) Treatment of metastatic melanoma: an overview. Oncology 23:488–496
de Giorgi V, Gori A, Alfaioli B, Papi F, Grazzini M, Rossari S, Lotti T, Massi D (2011) Influence of sex hormones on melanoma. J Clin Oncol 29:e94–e95; author reply e96. doi:10.1200/JCO.2010.33.1876
Janik ME, Bełkot K, Przybyło M (2014) Is oestrogen an important player in melanoma progression? Contemp Oncol 18:302–306. doi:10.5114/wo.2014.43938
Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS (1997) Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science 277:1508–1510
Watters JJ, Campbell JS, Cunningham MJ, Krebs EG, Dorsa DM (1997) Rapid membrane effects of steroids in neuroblastoma cells: effects of estrogen on mitogen activated protein kinase signalling cascade and c-fos immediate early gene transcription. Endocrinology 138:4030–4033. doi:10.1210/endo.138.9.5489
Filardo EJ, Quinn JA, Bland KI, Frackelton AR Jr (2000) Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol 14:1649–1660. doi:10.1210/mend.14.10.0532
Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA (2002) Mutations of the BRAF gene in human cancer. Nature 417:949–954. doi:10.1038/nature00766
Kanda N, Watanabe S (2003) 17β-estradiol inhibits oxidative stress-induced apoptosis in keratinocytes by promoting Bcl-2 expression. J Invest Dermatol 121:1500–1509. doi:10.1111/j.1523-1747.2003.12617.x
McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, Stivala F, Libra M, Basecke J, Evangelisti C, Martelli AM, Franklin RA (2007) Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta 1773:1263–1284. doi:10.1016/j.bbamcr.2006.10.001
Dobroff AS, Wang H, Melnikova VO, Villares GJ, Zigler M, Huang L, Bar-Eli M (2009) Silencing cAMP-response element-binding protein (CREB) identifies CYR61 as a tumor suppressor gene in melanoma. J Biol Chem 284:26194–26206. doi:10.1074/jbc.M109.019836
Hu W, Jin L, Jiang CC, Long GV, Scolyer RA, Wu Q, Zhang XD, Mei Y, Wu M (2013) AEBP1 upregulation confers acquired resistance to BRAF (V600E) inhibition in melanoma. Cell Death Dis 4:e914. doi:10.1038/cddis.2013.441
Johannessen CM, Johnson LA, Piccioni F, Townes A, Frederick DT, Donahue MK, Narayan R, Flaherty KT, Wargo JA, Root DE, Garraway LA (2013) A melanocyte lineage program confers resistance to MAP kinase pathway inhibition. Nature 504:138–142. doi:10.1038/nature12688
Travnickova-Bendova Z, Cermakian N, Reppert SM, Sassone-Corsi P (2002) Bimodal regulation of mPeriod promoters by CREB-dependent signaling and CLOCK/BMAL1 activity. Proc Natl Acad Sci USA 99:7728–7733. doi:10.1073/pnas.102075599
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100:57–70
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. doi:10.1016/j.cell.2011.02.013
Reardon JT, Sancar A (2006) Repair of DNA–polypeptide crosslinks by human excision nuclease. Proc Natl Acad Sci USA 103:4056–4061. doi:10.1073/pnas.0600538103
Menck CFM, Munford V (2014) DNA repair diseases: what do they tell us about cancer and aging? Genet Mol Biol 37:220–233
Kang TH, Lindsey-Boltz LA, Reardon JT, Sancar A (2010) Circadian control of XPA and excision repair of cisplatin-DNA damage by cryptochrome and HERC2 ubiquitin ligase. Proc Natl Acad Sci USA 107:4890–4895. doi:10.1073/pnas.0915085107
Kang TH, Reardon JT, Kemp M, Sancar A (2009) Circadian oscillation of nucleotide excision repair in mammalian brain. Proc Natl Acad Sci USA 106:2864–2867. doi:10.1073/pnas.0812638106
Wang Y, Cheng Y, Yu G, Jia B, Hu Z, Zhang L (2016) Expression of PER, CRY, and TIM genes for the pathological features of colorectal cancer patients. Onco Targets Ther 9:1997–2005. doi:10.2147/OTT.S96925
Cadenas C, van de Sandt L, Edlund K, Lohr M, Hellwig B, Marchan R, Schmidt M, Rahnenfuhrer J, Oster H, Hengstler JG (2014) Loss of circadian clock gene expression is associated with tumor progression in breast cancer. Cell Cycle 13:3282–3291. doi:10.4161/15384101.2014.954454
Fu L, Lee CC (2003) The circadian clock: pacemaker and tumor suppressor. Nat Rev Cancer 3:350–361. doi:10.1038/nrc1072
Acknowledgments
This work was partially supported by the Sao Paulo Research Foundation (FAPESP, Grant 2012/50214-4) and by the National Council of Technological and Scientific Development (CNPq, Grant 301293/2011-2 and 303070/2015-3). LVM de Assis and MN Moraes are fellows of FAPESP (2013/24337-4, 2014/16412-9, respectively).
Author information
Authors and Affiliations
Corresponding author
Additional information
Maristela Oliveira Poletini and Leonardo Vinicius Monteiro de Assis contributed equally to this work.
Rights and permissions
About this article
Cite this article
Poletini, M.O., de Assis, L.V.M., Moraes, M.N. et al. Estradiol differently affects melanin synthesis of malignant and normal melanocytes: a relationship with clock and clock-controlled genes. Mol Cell Biochem 421, 29–39 (2016). https://doi.org/10.1007/s11010-016-2781-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-016-2781-3