Abstract
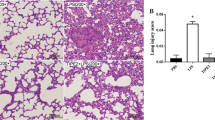
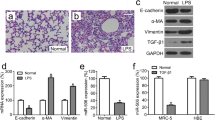
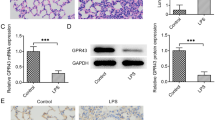
Acute respiratory distress syndrome (ARDS) is a common clinical disorder characterized by pulmonary edema leading to acute lung damage and arterial hypoxemia. Pulmonary fibrosis is a progressive, fibrotic lung disorder, whose pathogenesis in ARDS remains speculative. LincRNA-p21 was a novel regulator of cell proliferation, apoptosis and DNA damage response. This study aims to investigate the effects and mechanism of lincRNA-p21 on pulmonary fibrosis in ARDS. Purified 10 mg/kg LPS was dropped into airways of C57BL/6 mice. Expression levels of lincRNA-p21 and Thy-1 were measured by real-time PCR or western blotting. Proliferation of lung fibroblasts was analyzed by BrdU incorporation assay. Lung and BAL collagen contents were estimated using colorimetric Sircol assay. LincRNA-p21 expression was time-dependently increased and Thy-1 expression was time-dependently reduced in a mouse model of ARDS and in LPS-treated lung fibroblasts. Meanwhile, lung fibroblast proliferation was also time-dependently elevated in LPS-treated lung fibroblasts. In addition, lung fibroblast proliferation could be promoted by lincRNA-p21 overexpression and LPS treatment, however, the elevated lung fibroblast proliferation was further abrogated by Thy-1 overexpression or lincRNA-p21 interference. And Thy-1 interference could elevate cell viability of lung fibroblasts and rescue the reduction of lung fibroblast proliferation induced by lincRNA-p21 interference. Moreover, lincRNA-p21 overexpression dramatically inhibited acetylation of H3 and H4 at the Thy-1 promoter and Thy-1 expression levels in HLF1 cells. Finally, lincRNA-p21 interference rescued LPS-induced increase of lung and BAL collagen contents. LincRNA-p21 could lead to pulmonary fibrosis in ARDS by inhibition of the expression of Thy-1.






Similar content being viewed by others
References
Ware LB, Matthay MA (2000) The acute respiratory distress syndrome. N Engl J Med 342:1334–1349. doi:10.1056/NEJM200005043421806
Wheeler AP, Bernard GR (2007) Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet 369:1553–1564. doi:10.1016/S0140-6736(07)60604-7
Ware LB (2007) Clinical Year in Review III: asthma, lung transplantation, cystic fibrosis, acute respiratory distress syndrome. Proc Am Thorac Soc 4:489–493. doi:10.1513/pats.200707-102TT
Orme J Jr, Romney JS, Hopkins RO, Pope D, Chan KJ, Thomsen G, Crapo RO, Weaver LK (2003) Pulmonary function and health-related quality of life in survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med 167:690–694. doi:10.1164/rccm.200206-542OC
Katzenstein AL, Mukhopadhyay S, Myers JL (2008) Diagnosis of usual interstitial pneumonia and distinction from other fibrosing interstitial lung diseases. Hum Pathol 39:1275–1294. doi:10.1016/j.humpath.2008.05.009
Chida M, Ono S, Hoshikawa Y, Kondo T (2008) Subclinical idiopathic pulmonary fibrosis is also a risk factor of postoperative acute respiratory distress syndrome following thoracic surgery. Eur J Cardiothorac Surg 34:878–881. doi:10.1016/j.ejcts.2008.07.028
Robinson CM, Neary R, Levendale A, Watson CJ, Baugh JA (2012) Hypoxia-induced DNA hypermethylation in human pulmonary fibroblasts is associated with Thy-1 promoter methylation and the development of a pro-fibrotic phenotype. Respir Res 13:74. doi:10.1186/1465-9921-13-74
Fukuda Y, Ishizaki M, Masuda Y, Kimura G, Kawanami O, Masugi Y (1987) The role of intraalveolar fibrosis in the process of pulmonary structural remodeling in patients with diffuse alveolar damage. Am J Pathol 126:171–182
Ley B, Collard HR, King TE Jr (2011) Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 183:431–440. doi:10.1164/rccm.201006-0894CI
Zhou Y, Hagood JS, Lu B, Merryman WD, Murphy-Ullrich JE (2010) Thy-1-integrin alphav beta5 interactions inhibit lung fibroblast contraction-induced latent transforming growth factor-beta1 activation and myofibroblast differentiation. J Biol Chem 285:22382–22393. doi:10.1074/jbc.M110.126227
Bradley JE, Ramirez G, Hagood JS (2009) Roles and regulation of Thy-1, a context-dependent modulator of cell phenotype. BioFactors 35:258–265. doi:10.1002/biof.41
Rege TA, Hagood JS (2006) Thy-1, a versatile modulator of signaling affecting cellular adhesion, proliferation, survival, and cytokine/growth factor responses. Biochim Biophys Acta 1763:991–999. doi:10.1016/j.bbamcr.2006.08.008
Hagood JS, Prabhakaran P, Kumbla P, Salazar L, MacEwen MW, Barker TH, Ortiz LA, Schoeb T, Siegal GP, Alexander CB, Pardo A, Selman M (2005) Loss of fibroblast Thy-1 expression correlates with lung fibrogenesis. Am J Pathol 167:365–379. doi:10.1016/S0002-9440(10)62982-3
Sanders YY, Pardo A, Selman M, Nuovo GJ, Tollefsbol TO, Siegal GP, Hagood JS (2008) Thy-1 promoter hypermethylation: a novel epigenetic pathogenic mechanism in pulmonary fibrosis. Am J Respir Cell Mol Biol 39:610–618. doi:10.1165/rcmb.2007-0322OC
Sanders YY, Tollefsbol TO, Varisco BM, Hagood JS (2011) Epigenetic regulation of thy-1 by histone deacetylase inhibitor in rat lung fibroblasts. Am J Respir Cell Mol Biol 45:16–23. doi:10.1165/rcmb.2010-0154OC
Nicola T, Hagood JS, James ML, Macewen MW, Williams TA, Hewitt MM, Schwiebert L, Bulger A, Oparil S, Chen YF, Ambalavanan N (2009) Loss of Thy-1 inhibits alveolar development in the newborn mouse lung. Am J Physiol Lung Cell Mol Physiol 296:L738–L750. doi:10.1152/ajplung.90603.2008
Han Y, Liu Y, Gui Y, Cai Z (2013) Long intergenic non-coding RNA TUG1 is overexpressed in urothelial carcinoma of the bladder. J Surg Oncol 107:555–559. doi:10.1002/jso.23264
Yoon JH, Abdelmohsen K, Srikantan S, Yang X, Martindale JL, De S, Huarte M, Zhan M, Becker KG, Gorospe M (2012) LincRNA-p21 suppresses target mRNA translation. Mol Cell 47:648–655. doi:10.1016/j.molcel.2012.06.027
Zhou Y, Wu K, Jiang J, Huang J, Zhang P, Zhu Y, Hu G, Lang J, Shi Y, Hu L, Huang T, Kong X (2015) Integrative Analysis Reveals Enhanced Regulatory Effects of Human Long Intergenic Non-Coding RNAs in Lung Adenocarcinoma. J Genet Genomics 42:423–436. doi:10.1016/j.jgg.2015.07.001
Tang SS, Zheng BY, Xiong XD (2015) LincRNA-p21: implications in Human Diseases. Int J Mol Sci 16:18732–18740. doi:10.3390/ijms160818732
Ning Y, Yong F, Haibin Z, Hui S, Nan Z, Guangshun Y (2015) LincRNA-p21 activates endoplasmic reticulum stress and inhibits hepatocellular carcinoma. Oncotarget 6(29):28151–28163
Wang G, Li Z, Zhao Q, Zhu Y, Zhao C, Li X, Ma Z, Li X, Zhang Y (2014) LincRNA-p21 enhances the sensitivity of radiotherapy for human colorectal cancer by targeting the Wnt/beta-catenin signaling pathway. Oncol Rep 31:1839–1845. doi:10.3892/or.2014.3047
Zhai H, Fesler A, Schee K, Fodstad O, Flatmark K, Ju J (2013) Clinical significance of long intergenic noncoding RNA-p21 in colorectal cancer. Clin Colorectal Cancer 12:261–266. doi:10.1016/j.clcc.2013.06.003
He C, Ding JW, Li S, Wu H, Jiang YR, Yang W, Teng L, Yang J, Yang J (2015) The role of long intergenic Noncoding RNA p21 in vascular endothelial cells. DNA Cell Biol. doi:10.1089/dna.2015.2966
Hall JR, Messenger ZJ, Tam HW, Phillips SL, Recio L, Smart RC (2015) Long noncoding RNA lincRNA-p21 is the major mediator of UVB-induced and p53-dependent apoptosis in keratinocytes. Cell Death Dis 6:e1700. doi:10.1038/cddis.2015.67
Huarte M, Guttman M, Feldser D, Garber M, Koziol MJ, Kenzelmann-Broz D, Khalil AM, Zuk O, Amit I, Rabani M (2010) A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell 142:409–419
Xia H, Diebold D, Nho R, Perlman D, Kleidon J, Kahm J, Avdulov S, Peterson M, Nerva J, Bitterman P, Henke C (2008) Pathological integrin signaling enhances proliferation of primary lung fibroblasts from patients with idiopathic pulmonary fibrosis. J Exp Med 205:1659–1672. doi:10.1084/jem.20080001
Meijerink J, Mandigers C, van de Locht L, Tonnissen E, Goodsaid F, Raemaekers J (2001) A novel method to compensate for different amplification efficiencies between patient DNA samples in quantitative real-time PCR. J Mol Diagn 3:55–61. doi:10.1016/S1525-1578(10)60652-6
Rege TA, Hagood JS (2006) Thy-1 as a regulator of cell-cell and cell-matrix interactions in axon regeneration, apoptosis, adhesion, migration, cancer, and fibrosis. FASEB J 20:1045–1054. doi:10.1096/fj.05-5460rev
Xing S, Nie F, Xu Q, Deng Y, Li W, Yang Z, Zhao X, Zhu P, Wang X, Gao Y, He Z (2015) HDAC is essential for epigenetic regulation of Thy-1 gene expression during LPS/TLR4-mediated proliferation of lung fibroblasts. Lab Invest. doi:10.1038/labinvest.2015.97
Bao X, Wu H, Zhu X, Guo X, Hutchins AP, Luo Z, Song H, Chen Y, Lai K, Yin M, Xu L, Zhou L, Chen J, Wang D, Qin B, Frampton J, Tse HF, Pei D, Wang H, Zhang B, Esteban MA (2015) The p53-induced lincRNA-p21 derails somatic cell reprogramming by sustaining H3K9me3 and CpG methylation at pluripotency gene promoters. Cell Res 25:80–92. doi:10.1038/cr.2014.165
Matthay MA, Zimmerman GA (2005) Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am J Respir Cell Mol Biol 33:319–327. doi:10.1165/rcmb.F305
London L, Majeski EI, Altman-Hamamdzic S, Enockson C, Paintlia MK, Harley RA, London SD (2002) Respiratory reovirus 1/L induction of diffuse alveolar damage: pulmonary fibrosis is not modulated by corticosteroids in acute respiratory distress syndrome in mice. Clin Immunol 103:284–295
Katzenstein AL, Myers JL (1998) Idiopathic pulmonary fibrosis: clinical relevance of pathologic classification. Am J Respir Crit Care Med 157:1301–1315. doi:10.1164/ajrccm.157.4.9707039
Neveu WA, Mills ST, Staitieh BS, Sueblinvong V (2015) TGFbeta1 epigenetically modifies Thy-1 expression in primary lung fibroblasts. Am J Physiol Cell Physiol 00086:2015. doi:10.1152/ajpcell.00086.2015
Meltzer EB, Noble PW (2008) Idiopathic pulmonary fibrosis. Orphanet J Rare Dis 3:8. doi:10.1186/1750-1172-3-8
Ramos C, Montano M, Garcia-Alvarez J, Ruiz V, Uhal BD, Selman M, Pardo A (2001) Fibroblasts from idiopathic pulmonary fibrosis and normal lungs differ in growth rate, apoptosis, and tissue inhibitor of metalloproteinases expression. Am J Respir Cell Mol Biol 24:591–598. doi:10.1165/ajrcmb.24.5.4333
Hardie WD, Glasser SW, Hagood JS (2009) Emerging concepts in the pathogenesis of lung fibrosis. Am J Pathol 175:3–16. doi:10.2353/ajpath.2009.081170
Sun L, Luo H, Liao Q, Bu D, Zhao G, Liu C, Liu Y, Zhao Y (2013) Systematic study of human long intergenic non-coding RNAs and their impact on cancer. Sci China Life Sci 56:324–334. doi:10.1007/s11427-013-4460-x
Huarte M, Guttman M, Feldser D, Garber M, Koziol MJ, Kenzelmann-Broz D, Khalil AM, Zuk O, Amit I, Rabani M, Attardi LD, Regev A, Lander ES, Jacks T, Rinn JL (2010) A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell 142:409–419. doi:10.1016/j.cell.2010.06.040
Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, Wang Y, Brzoska P, Kong B, Li R, West RB, van de Vijver MJ, Sukumar S, Chang HY (2010) Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 464:1071–1076. doi:10.1038/nature08975
Baldassarre A, Masotti A (2012) Long non-coding RNAs and p53 regulation. Int J Mol Sci 13:16708–16717. doi:10.3390/ijms131216708
Wu G, Cai J, Han Y, Chen J, Huang ZP, Chen C, Cai Y, Huang H, Yang Y, Liu Y, Xu Z, He D, Zhang X, Hu X, Pinello L, Zhong D, He F, Yuan GC, Wang DZ, Zeng C (2014) LincRNA-p21 regulates neointima formation, vascular smooth muscle cell proliferation, apoptosis, and atherosclerosis by enhancing p53 activity. Circulation 130:1452–1465. doi:10.1161/CIRCULATIONAHA.114.011675
Dimitrova N, Zamudio JR, Jong RM, Soukup D, Resnick R, Sarma K, Ward AJ, Raj A, Lee JT, Sharp PA, Jacks T (2014) LincRNA-p21 activates p21 in cis to promote Polycomb target gene expression and to enforce the G1/S checkpoint. Mol Cell 54:777–790. doi:10.1016/j.molcel.2014.04.025
McIntosh JC, Hagood JS, Richardson TL, Simecka JW (1994) Thy1 (+) and (−) lung fibrosis subpopulations in LEW and F344 rats. Eur Respir J 7:2131–2138
Sanders YY, Kumbla P, Hagood JS (2007) Enhanced myofibroblastic differentiation and survival in Thy-1(−) lung fibroblasts. Am J Respir Cell Mol Biol 36:226–235. doi:10.1165/rcmb.2006-0178OC
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests financial or nonfinancial related to the content of this article.
Rights and permissions
About this article
Cite this article
Zhou, Wq., Wang, P., Shao, Qp. et al. Lipopolysaccharide promotes pulmonary fibrosis in acute respiratory distress syndrome (ARDS) via lincRNA-p21 induced inhibition of Thy-1 expression. Mol Cell Biochem 419, 19–28 (2016). https://doi.org/10.1007/s11010-016-2745-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-016-2745-7