Abstract
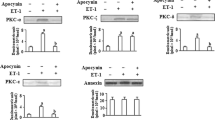
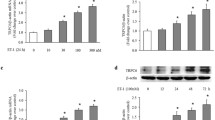
Treatment of bovine pulmonary artery smooth muscle cells with endothelin-1 (ET-1) caused an increase in the expression and activation of proMMP-2 in the cells. The present study was undertaken to determine the underlying mechanisms involved in this scenario. We demonstrated that (i) pretreatment with NADPH oxidase inhibitor, apocynin; PKC-α inhibitor, Go6976; p38MAPK inhibitor SB203580 and NF-κB inhibitor, Bay11-7082 inhibited the expression and activation of proMMP-2 induced by ET-1; (ii) ET-1 treatment to the cells stimulated NADPH oxidase and PKCα activity, p38MAPK phosphorylation as well as NF-κB activation by translocation of NF-κBp65 subunit from cytosol to the nucleus, and subsequently by increasing its DNA-binding activity; (iii) ET-1 increases MT1-MMP expression, which was inhibited upon pretreatment with apocynin, Go6976, SB293580, and Bay 11-7082; (iv) ET-1 treatment to the cells downregulated TIMP-2 level. Although apocynin and Go6976 pretreatment reversed ET-1 effect on TIMP-2 level, yet pretreatment of the cells with SB203580 and Bay 11-7082 did not show any discernible change in TIMP-2 level by ET-1. Overall, our results suggest that ET-1-induced activation of proMMP-2 is mediated via cross-talk between NADPH oxidase-PKCα-p38MAPK and NFκB-MT1MMP signaling pathways along with a marked decrease in TIMP-2 expression in the cells.











Similar content being viewed by others
Abbreviations
- SMC:
-
Smooth muscle cell
- proMMP-2:
-
Pro matrix metalloprotease 2
- MT1-MMP:
-
Membrane type 1 matrix metalloprotease
- TIMP-2:
-
Tissue inhibitor of matrix metalloprotease 2
- PKC-α:
-
Protein kinase C α
- IKK:
-
Inhibitory κB kinase
- NF-κB:
-
Nuclear factor κB, IκB-α, inhibitory κBα
References
Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF (2009) Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol 297:L1013–L1032
Vieillard-Baron A, Frisdal E, Raffestin B, Baker AH, Eddahibi S, Adnot S, D’Ortho MP (2003) Inhibition of matrix metalloproteinases by lung TIMP-1 gene transfer limits monocrotaline-induced pulmonary vascular remodeling in rats. Hum Gene Ther 14:861–869
Stenmark KR, Mecham RP (1997) Cellular and molecular mechanisms of pulmonary vascular remodeling. Ann Rev Physiol 59:89–144
Stamenkovic I (2003) Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol 200:448–464
Woessner JF Jr (1991) Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J 5:2145–2154
Jo Y, Yeon J, Kim HJ, Lee ST (2000) Analysis of tissue inhibitor of metalloproteinases-2 effect on pro-matrix metalloproteinase-2 activation by membrane-type 1 matrix metalloproteinase using baculovirus/insect-cell expression system. Biochem J 345:511–519
Strongin AY, Collier I, Bannikov G, Marmer BL, Grant GA, Charpiot P (1995) Mechanism of cell surface activation of 72 kDa type IV collagenase. Isolation of the activated form of the membrane metalloproteinase. J Biol Chem 270:5331–5338
Morrison CJ, Butler GS, Bigg HF, Roberts CR, Soloway PD, Overall CM (2001) Cellular activation of MMP-2 (gelatinase-A) by MT2.-MMP occurs via a TIMP-2 independent pathway. J Biol Chem 276:47402–47410
Han YP, Tuan TL, Wu H, Hughes M, Garner WL (2001) TNF-α stimulates activation of proMMP-2 in human skin through NF-κB mediated induction of MT1-MMP. J Cell Sci 114:131–139
Crabbe T, Smith B, O’Connell J, Docherty A (1994) Human progelatinase A can be activated by matrilysin. FEBS Lett 345:14–16
Koo BH, Park MY, Jeon OH, Kim DS (2009) Regulatory mechanism of matrix metalloprotease-2 enzymatic activity by factor Xa and thrombin. J Biol Chem 284:23375–23385
Le DM, Besson A, Fogg DK, Choi KS, Waisman DM, Goodyer CG, Rewcastle B, Yong VW (2003) Exploitation of astrocytes by glioma cells to facilitate invasiveness: a mechanism involving matrix metalloproteinase-2 and the urokinase-type plasminogen activator protein cascade. J Neurosci 23:4034–4043
Pezzato E, Done M, Sartor L, Bell Alice I, Benelli R, Albini A, Garbisa S (2003) Proteinase-3 directly activates MMP-2 and degrades gelatine and Matrigel: differential inhibition by (−) epigallocatechin-3-gallate. J Leukocy Biol 74:88–94
Chen JM, Fortunato M, Stevens RA, Barrett A (2001) Activation of progelatinase A by mammalian legumain, a recently discovered cysteine protease. Biol Chem 382:777–783
Kishi K, Muramatsu M, Jin D, Furabayashi K, Takai S, Tania H, Miyazaki M (2007) The effects of chymase on matrix metalloproteinase-2 activation in neointimal hyperplasia after balloon injury in dogs. Hyperten Res 30:77–83
Lambeth JD (2004) NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 4:181–189
Lassegue B, Clempus RE (2003) Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol 285:R277–R297
Wedgwood S, Black SM (2003) Role of reactive oxygen species in vascular remodeling associated with pulmonary hypertension. Antioxid Redox Signal 5:759–769
Luchtefeld M, Grote K, Grothusen C, Bley S, Bandlow N, Selle T, Strüber M, Haverich A, Bavendiek U, Drexler H, Schieffer B (2005) Angiotensin II induces MMP-2 in a p47phox-dependent manner. Biochem Biophys Res Commun 328:183–188
Kim KH, Cho YS, Park JM, Yoon SO, Kim KW, Chung AS (2007) Pro-MMP-2 activation by the PPARgamma agonist, ciglitazone, induces cell invasion through the generation of ROS and the activation of ERK. FEBS Lett 581:3303–3310
Chakraborti S, Michael JR (1993) Oxidant-mediated activation of phospholipase A2 in rabbit pulmonary arterial smooth muscle cells. Mol Cell Biochem 122:9–15
Korchak HM, Rossi MW, Kilpatrick LE (1998) Selective role for beta-protein kinase C in signaling for O-2 generation but not degranulation or adherence in differentiated HL60 cells. J Biol Chem 16:27292–27299
Wang Y, Biswas G, Prabu SK, Avadhani NG (2006) Modulation of mitochondrial metabolic function by phorbol 12-myristate 13-acetate through increased mitochondrial translocation of protein kinase Calpha in C2C12 myocytes. Biochem Pharmacol 28:881–892
Gopalakrishna R, Jaken S (2000) Protein kinase C signaling and oxidative stress. Free Radic Biol Med 9:1349–1361
DelCarlo M, Loeser RF (2006) Chondrocyte cell death mediated by reactive oxygen species-dependent activation of PKC-betaI. Am J Physio. Cell Physiol 290:802–811
Chakraborti S, Roy S, Mandal A, Dey K, Chowdhury A, Shaikh S, Chakraborti T (2012) Role of PKCα-p(38)MAPK-G(i)α axis in NADPH oxidase derived O(2)(–)-mediated activation of cPLA(2) under U46619 stimulation in pulmonary artery smooth muscle cells. Arch Biochem Biophys 523:169–180
Roy S, Chakraborti T, Chowdhury A, Chakraborti S (2013) Role of PKC-α in NF-κB-MT1-MMP-mediated activation of proMMP-2 by TNF-α in pulmonary artery smooth muscle cells. J Biochem 153:289–302
Pons M, Cousins SW, Alcazar O, Striker GE, Marin-Castaño ME (2011) Angiotensin II-induced MMP-2 activity and MMP-14 and basigin protein expression are mediated via the angiotensin II receptor type 1-mitogen-activated protein kinase 1 pathway in retinal pigment epithelium: implications for age-related macular degeneration. Am J Pathol 178:2665–2681
Denkert C, Siegert A, Leclere A, Turzynski A, Hauptmann S (2002) An inhibitor of stress-activated MAP-kinases reduces invasion and MMP-2 expression of malignant melanoma cells. Clin Exp Metastasis 19:79–85
Carter AB, Knudtson KL, Monick MM, Hunninghake GW (1999) The p38 mitogen-activated protein kinase is required for NF-kappaB-dependent gene expression. The role of TATA-binding protein (TBP). J Biol Chem 274:30858–30863
Han YP, Tuan TL, Wu H, Hughes M, Garner WL (2001) TNF-alpha stimulates activation of pro-MMP2 in human skin through NF-(kappa) B mediated induction of MT1-MMP. J Cell Sci 114:131–139
Melnikova VO, Mourad-Zeidan AA, Lev DC, Bar-Eli M (2006) Platelet-activating factor mediates MMP-2 expression and activation via phosphorylation of cAMP-response element-binding protein and contributes to melanoma metastasis. J Biol Chem 281:2911–2922
Chen F, Castranova V, Shi X, Demers LM (1999) New insights into the role of nuclear factor-kappaB, a ubiquitous transcription factor in the initiation of diseases. Clin Chem 45:7–17
Wilson JL, Yu J, Taylor L, Polgar P (2015) Hyperplastic Growth of Pulmonary Artery Smooth Muscle Cells from Subjects with Pulmonary Arterial Hypertension Is Activated through JNK and p38 MAPK. PLoS ONE 10:e0123662
Farkas D, Alhussaini AA, Kraskauskas D, Kraskauskiene V, Cool CD, Nicolls MR, Natarajan R, Farkas L (2014) Nuclear factor κB inhibition reduces lung vascular lumen obliteration in severe pulmonary hypertension in rats. Am J Respir Cell Mol Biol 51:413–425
Deuchar GA, Docherty A, MacLean MR, Hicks MN (2002) Pulmonary hypertension secondary to left ventricular dysfunction: the role of nitric oxide and endothelin-1 in the control of pulmonary vascular tone. Br J Pharmacol 35:1060–1068
Stewart DJ, Levy RD, Cernacek P, Langleben D (1991) Increased plasma endothelin-1 in pulmonary hypertension: marker or mediator of disease? Ann Intern Med 114:464–469
Wedgwood S, Dettwan RW, Blach SM (2001) ET-1 stimulates pulmonary arterial smooth muscle cell proliferation via induction of reactive oxygen species. Am J Physiol 281:1056–1067
Sun XZ, Lin Y, Fang P, Li MX (2010) Inhibition of cGMP phosphodiesterase 5 suppresses matrix metalloproteinase-2 production in pulmonary artery smooth muscle cells. Clin Exp Pharmacol Physiol 37:362–367
Wort SJ, Ito M, Chou PC, Mc Master SK, Badiger R, Jazrawi E, de Souza P, Evans TW, Mitchell JA, Pinhu L, Ito K, Adcock IM (2009) Synergistic induction of endothelin-1 by tumor necrosis factor alpha and interferon gamma is due to enhanced NF-kappaB binding and histone acetylation at specific kappaB sites. J Biol Chem 284:24297–24305
Quchenberger P, Bierhaus A, Fasching P, Muellner C, Klevesath M, Hong M, Stier G, Sattler M, Schleicler E, Speiser W, Nawrath PP (2009) Endothelin-1 transcription is controlled by nuclear factor kappa-B in AGE stimulated cultured endothelial cells. Diabetes 49:1561–1570
Chakraborti S, Mandal A, Das S, Chakraborti T (2004) Inhibition of Na+/Ca2+ exchanger by peroxynitrite in microsomes of pulmonary artery smooth muscle: role of matrix metalloproteinase-2. Biochim Biophys Acta 1671:70–78
You R, Zheng M, McKeown-Longo PJ (2010) The first type III repeat in fibronectin activates an inflammatory pathway in dermal fibroblasts. J Biol Chem 285:36255–36259
Hua J, Hasebe T, Someya A, Nakamura S, Sugimoto K, Nagaoka I (2000) Evaluation of the expression of NADPH oxidase components during maturation of HL-60 cells to neutrophil lineage. J Leukoc Biol 68:216–224
Das S, Mandal M, Chakraborti T, Mandal A, Chakraborti S (2004) Isolation of MMP-2 from MMP-2/TIMP-2 complex: characterization of the complex and the free enzyme in pulmonary vascular smooth muscle plasma membrane. Biochim Biophys Acta 1674:158–174
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci (USA) 76:4350–4354
Chowdhury A, Roy S, Chakraborti T, Dey K, Chakraborti S (2014) Activation of proMMP-2 by U46619 occurs via involvement of p(38)MAPK-NFκB-MT1MMP signaling pathway in pulmonary artery smooth muscle cells. Mol Cell Biochem 385:53–68
Lafleur MA, Forsyth PA, Atkinson SJ, Murphy G, Edwards DR (2001) Perivascular cells regulate endothelial membrane type-1 matrix metalloproteinase activity. Biochem Biophys Res Commun 282:463–473
Lenardo MJ, Kuang A, Gifford A, Baltimore D (1988) NF-kappa B protein purification from bovine spleen: nucleotide stimulation and binding site specificity. Proc Natl Acad Sci (USA) 85:8825–8829
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85
Chakrabarti S, Patel KD (2005) Matrix metalloproteinase-2 (MMP-2) and MMP-9 in pulmonary pathology. Exp Lung Res 31:599–621
Beswick RA, Dorrance AM, Leite R, Webb RC (2001) NADH/NADPH oxidase and enhanced superoxide production in the mineralocorticoid hypertensive rat. Hypertension 38:1107–1111
Meyer JW, Schmitt ME (2000) A central role for the endothelial NADPH oxidase in atherosclerosis. FEBS Lett 472:1–4
Bolla M, Matrougui K, Loufrani L, Maclouf J, Levy B, Levy-Toledano S, Habib A, Henrion D (2002) p38 mitogen-activated protein kinase activation is required for thromboxane-induced contraction in perfused and pressurized rat mesenteric resistance arteries. J Vasc Res 39:353–360
Kwon CH, Moon HJ, Park HJ, Choi JH, do Park Y (2013) S100A8 and S100A9 promotes invasion and migration through p38 mitogen-activated protein kinase-dependent NF-κB activation in gastric cancer cells. Mol Cells 35:226–234
Juliana C, Fernandes-Alnemri T, Wu J, Datta P, Solorzano L, Yu JW, Meng R, Quong AA, Latz E, Scott CP, Alnemri ES (2010) Anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome. J Biol Chem 285:9792–9802
Lu KV, Jong KA, Rajasekaran AK, Cloughesy TF, Mischel PS (2004) Up regulation of tissue inhibitor of metalloproteinases (TIMP)-2 promotes matrix metalloproteinase (MMP)-2 activation and cell invasion in a human glioblastoma cell line. Lab Invest 84:8–20
Park JM, Kim A, Oh JH, Chung AS (2007) Methylseleninic acid inhibits PMA-stimulated pro-MMP-2 activation mediated by MT1-MMP expression and further tumor invasion through suppression of NF-kappaB activation. Carcinogenesis 28:837–847
Michael JR, Markewitz BA (1996) Endothelins and the lung. Am J Respir Crit Care Med 154:555–581
Frisdal E, Gest V, Vieillard-Baron A, Levame M, Lepetit H, Eddahibi S, Lafuma C, Harf A, Adnot S, d’Ortho MP (2001) Gelatinase expression in pulmonary arteries during experimental pulmonary hypertension. Eur Respir J 18:838–845
Acknowledgments
Financial assistance from the Council for Scientific and Industrial Research (CSIR, New Delhi) and University grants commission (UGC, New Delhi) are greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sarkar, J., Chowdhury, A., Chakraborti, T. et al. Cross-talk between NADPH oxidase-PKCα-p38MAPK and NF-κB-MT1MMP in activating proMMP-2 by ET-1 in pulmonary artery smooth muscle cells. Mol Cell Biochem 415, 13–28 (2016). https://doi.org/10.1007/s11010-016-2673-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-016-2673-6