Abstract
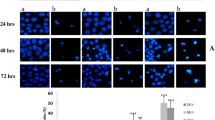
To investigate whether endoplasmic reticulum (ER) stress participates in the induction of apoptosis in HepG2 cells exposed to high glucose and explore its probable mechanism. A series of experiments were performed following HepG2 cells treated with different concentrations of glucose for 48 h. The apoptosis was detected by means of Hoechst staining and flow cytometry. Caspase-3 activity assay was performed by measuring the pNA (p-nitroaniline) to indirectly reveal the catalytic activity of caspase-3. The expression levels of apoptosis-, ER stress-associated proteins and MAPKs were analyzed by western blot. To further characterize the molecular mechanisms, the effects of antioxidant alpha-lipoic acid (ALA) and specific inhibitors for JNK and p38 (SP600125 and SB203580, respectively) were examined by Hoechst staining, immunofluorescence, and western blot. After HepG2 cells were incubated with high glucose for 48 h, both Hoechst staining and flow cytometry analyses unveiled the apoptosis of HepG2 cells. Caspase-3 activity assay revealed that the activity of caspase-3 was enhanced. Western blot showed an enhancement of pro-caspase-9 degradation, a reduction of Bcl-2/Bax ratio, a decrease in GRP78 expression, and increases in CHOP and p47/phox levels. In addition, western blot analysis presented that phosphorylation of p38 and JNK was triggered and that the expression of ASK1 was elevated. In the case of the contributions of oxidative stress and the MAPK signaling pathways, all ALA, SP600125 and SB203580 were able to largely rescue high glucose-induced apoptosis. High glucose induced the apoptosis in HepG2 cells through the activation of ASK1-p38/JNK pathway mediated by ER stress and oxidative stress.








Similar content being viewed by others
References
Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M (2011) Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose) National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country- years and 2.7 million participants. Lancet 378(9785):31–40
Global status report on noncommunicable diseases (2010) Geneva, World Health Organization
Liu BC, Song X, Lu XY, Li DT, Eaton DC, Shen BZ, Li XQ, Ma HP (2013) High glucose induces podocyte apoptosis by stimulating TRPC6 via elevation of reactive oxygen species. Biochim Biophys Acta 1833:1434–1442
Lakshmanana AP, Harimaa M, Suzukib K, Soetiknoa V, Nagatac M, Nakamuraa T, Takahashid T, Sonee H, Kawachif H, Watanabea K (2013) The hyperglycemia stimulated myocardial endoplasmic reticulum (ER) stress contributes to diabetic cardiomyopathy in the transgenic non-obese type 2 diabetic rats: a differential role of unfolded protein response (UPR) signaling proteins. Int J Bioche Cell Biol 45:438–447
Villarroel M, Garcia-Ramirez M, Corraliza L, Hernandez C, Simo R (2009) Effects of high glucose concentration on the barrier function and the expression of tight junction proteins in human retinal pigment epithelial cells. Exp Eye Res 89:913–920
Zhang KZ (2010) Integration of ER stress, oxidative stress and the inflammatory response in health and disease. Int J Clin Exp Med 3(1):33–40
Hayden MR, Tyagi SC, Kerklo MM, Nicolls MR (2005) Type 2 diabetes mellitus as a conformational disease. JOP 6:287–302
Szegezdi E, Logue SE, Gorman AM, Samali A (2006) Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep 7:880–885
Sari FR, Widyantoro B, Thandavarayan RA, Harima M, Lakshmanan AP, Zhang SS, Muslin AJ, Suzuki K, Kodama M, Watanabe K (2011) Attenuation of CHOP-mediated myocardial apoptosis in pressure-overloaded dominant negative p38α mitogen-activated protein kinase mice. Cell Physiol Biochem 27:487–496
Lakshmanan AP, Thandavarayan RA, Palaniyandi SS, Sari FR, Meilei H, Giridharan VV, Soetikno V, Suzuki K, Kodama M, Watanabe K (2011) Modulation of AT-1R/CHOP-JNK-Caspase12 pathway by olmesartan treatment attenuates ER stress-induced renal apoptosis in streptozotocin-induced diabetic mice. Eur J Pharm Sci 44:627–634
Liang SH, Zhang W, McGrath BC, Zhang P, Cavener DR (2006) PERK (eIF2alpha kinase) is required to activate the stress-activated MAPKs and induce the expression of immediate-early genes upon disruption of ER calcium homoeostasis. Biochem J 393:201–209
Logue SE, Cleary P, Saveljeva S, Samali A (2013) New directions in ER stress-induced cell death. Apoptosis 18(5):537–546
Matsukawa J, Matsuzawa A, Takeda K, Ichijo H (2004) The ASK1-MAP kinase cascades in mammalian stress response. J Biochem 136:261–265
Cargnello M, Roux PP (2011) Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev 75(1):50–83
Junttila MR, Li SP, Westermarck J (2008) Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J 22:954–965
Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, Minowa O, Miyazono K, Noda T, Ichijo H (2001) ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep 2(3):222–228
Nishitoh H, Matsuzawa A, Tobiume K, Saegusa K, Takeda K, Inoue K, Hori S, Kakizuka A, Ichijo H (2002) ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev 16:1345–1355
Nishitoh H, Kadowaki H, Nagai A, Maruyama T, Yokota T, Fukutomi H, Noguchi T, Matsuzawa A, Takeda K, Ichijo H (2008) ALS-linked mutant SOD1 induces ER stress- and ASK1-dependent motor neuron death by targeting Derlin-1. Genes Dev 22:1451–1464
Hetz CA (2007) ER stress signaling and the BCL-2 family of proteins: from adaptation to irreversible cellular damage. Antioxid Redox Signal 9(12):2345–2355
Nemcova-Furstova V, Balusikova K, Sramek J, James RF, Kovar J (2013) Caspase-2 and JNK activated by saturated fatty acids are not involved in apoptosis induction but modulate ER stress in human pancreatic β-cells. Cell Physiol Biochem 31(2–3):277–289
Gu XM, Li K, Laybutt DR, He ML, Zhao HL, Chan JCN, Xu G (2010) Bip overexpression, but not CHOP inhibition, attenuates fatty-acid-induced endoplasmic reticulum stress and apoptosis in HepG2 liver cells. Life Sci 87:724–732
Zhang LL, Lai E, Teodoro T, Volchuk A (2009) GRP78, but not protein-disulfide isomerase, partially reverses hyperglycemia-induced inhibition of insulin synthesis and secretion in pancreatic β-cells. J Biol Chem 284(8):5289–5298
Mazumder S, Plesca D, Almasan A (2008) Caspase-3 activation is a critical determinant of genotoxic stress–induced apoptosis. Methods Mol Biol 414:13–21
Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8(7):519–529
Chandrasekaran K, Swaminathan K, Chatterjee S, Dey A (2010) Apoptosis in HepG2 cells exposed to high glucose. Toxicol In Vitro 24(2):387–396
Matsuzawa A, Nishitoh H, Tobiume K, Takeda K, Ichijo H (2002) Physiological Roles of ASK1-Mediated Signal Transduction in Oxidative Stress- and Endoplasmic Reticulum Stress-Induced Apoptosis: advanced Findings from ASK1 Knockout Mice. Antioxid Redox Signal 4(3):415–425
Nagata Y, Todokoro K (1999) Requirement of activation of JNK and p38 for environmental stress-induced erythroid differentiation and apoptosis and of inhibition of ERK for apoptosis. Blood 94(3):853–863
Evans JL, Goldfine ID, Maddux BA, Grodsky GM (2002) Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev 23(5):599–622
Deursen DV, Jansen H, Verhoeven AJM (2008) Glucose increases hepatic lipase expression in HepG2 liver cells through upregulation of upstream stimulatory factors 1 and 2. Diabetologia 51:2078–2087
Okada K, Minamino T, Tsukamoto Y, Liao Y, Tsukamoto O, Takashima S, Hirata A, Fujita M, Nagamachi Y, Nakatani T, Yutani C, Ozawa K, Ogawa S, Tomoike H, Hori M, Kitakaze M (2004) Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation 110:705–712
Zhao LH, Ackerman SL (2006) Endoplasmic reticulum stress in health and disease. Curr Opin Cell Biol 18(4):444–452
Yamagishi N, Ueda T, Mori A, Saito Y, Hatayama T (2012) Decreased expression of endoplasmic reticulum chaperone GRP78 in liver of diabetic mice. Biochem Biophys Res Commun 417:364–370
Oyadomari S, Mori M (2004) Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ 11:381–389
Zinszner H, Kuroda M, Wang XZ, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D (1998) CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev 12:982–995
Stankovic MN, Mladenovic D, Ninkovic M, Duricic I, Sobajic S, Jorgacevic B, Luka S, Vukicevic RJ, Radosavljevic TS (2014) The effects of a-lipoic acid on liver oxidative stress and free fatty acid composition in methionine-choline deficient diet-induced NAFLD. J Med Food 17(2):254–261
Elshazly SM, El-Moselhy MA, Barakat W (2014) Insights in the mechanism underlying the protective effect of α-lipoic acid against acetaminophen-hepatotoxicity. Eur J Pharmacol 726:116–123
Kim MY, Kim EJ, Kim YN, Choi C, Lee BH (2011) Effects of α-lipoic acid and L-carnosine supplementation on antioxidant activities and lipid profiles in rats. Nutr Res Pract 5:421–428
Shay KP, Moreau RF, Smith EJ, Smith AR, Hagen TM (2009) Alpha-lipoic acid as a dietary supplement: molecular mechanisms and therapeutic potential. Biochim Biophys Acta 1790:1149–1160
Hahm JR, Noh HS, Ha JH, Roh GS, Kim DR (2014) Alpha-lipoic acid attenuates adipocyte differentiation and lipid accumulation in 3T3-L1 cells via AMPK-dependent autophagy. Life Sci 100(2):125–132
Hinson JA, Pumford NR, Roberts DW (1995) Mechanisms of acetaminophen toxicity: immunochemical detection of drug-protein adducts. Drug Metab Rev 27(1–2):73–92
Rochette L, Ghibu S, Richard C, Zeller M, Cottin Y, Vergely C (2013) Direct and indirect antioxidant properties of α-lipoic acid and therapeutic potential. Mol Nutr Food Res 57:114–125
Higa A, Chevet E (2012) Redox signaling loops in the unfolded protein response. Cell Signal 24(8):1548–1555
Glover-Cutter KM, Lin S, Blackwell TK (2013) Integration of the unfolded protein and oxidative stress responses through SKN-1/Nrf. PLoS Genet 9(9):e1003701. doi:10.1371/journal.pgen.1003701
Liu HB, Xiao YL, Xiong CM, Wei AH, Ruan JL (2011) Apoptosis induced by a new flavonoid in human hepatoma HepG2 cells involves reactive oxygen species-mediated mitochondrial dysfunction and MAPK activation. Eur J Pharmacol 654:209–216
Pate T, Gores GJ, Kaufmann SH (1996) The role of proteases during apoptosis. FASEB J 10:587–597
Rojas-Rivera D, Caballero B, Zamorano S, Lisbona F, Hetz C (2010) Alternative functions of the BCL-2 protein family at the endoplasmic reticulum. Adv Exp Med Biol 687:33–47
Ghribi O, DeWitt DA, Forbes MS, Herman MM, Savory J (2001) Co-involvement of mitochondria and endoplasmic reticulum in regulation of apoptosis: changes in cytochrome c, Bcl-2 and Bax in the hippocampus of aluminum-treated rabbits. Brain Res 903(1–2):66–73
Korsmeyer SJ, Yin XM, Oltvai ZN, Veis-Novack DJ, Linette GP (1995) Reactive oxygen species and the regulation of cell death by the Bcl-2 gene family. Biochim Biophys Acta 1271(1):63–66
Roux PP, Blenis J (2004) ERK and p38 MAPK-Activated Protein Kinases: a Family of Protein Kinases with Diverse Biological Functions. Microbiol Mol Biol Rev 68(2):320–344
Xia ZG, Dickens M, Raingeaud J, Davis RJ, Greenberg ME (1995) Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270(5240):1326–1331
Szydlowska K, Gozdz A, Dabrowski M, Zawadzka M, Kaminska B (2010) Prolonged activation of ERK triggers glutamate-induced apoptosis of astrocytes: neuroprotective effect of FK506. J Neurochem 113(4):904–918
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 81470568).
Author information
Authors and Affiliations
Corresponding author
Additional information
Qiaoling Jiang and Yujun Yuan contributed equally to this work.
Rights and permissions
About this article
Cite this article
Jiang, Q., Yuan, Y., Zhou, J. et al. Apoptotic events induced by high glucose in human hepatoma HepG2 cells involve endoplasmic reticulum stress and MAPK’s activation. Mol Cell Biochem 399, 113–122 (2015). https://doi.org/10.1007/s11010-014-2238-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-014-2238-5