Abstract
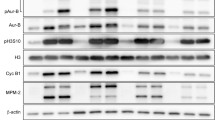
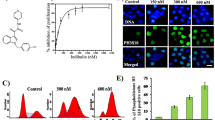
Dolastatin 15 (DL15) is a potent, tubulin-targeted, vinca-site binding, anticancer agent that induces mitotic arrest and inhibit cell proliferation in a variety of cell types. Several analogs of DL15, including LU 103793 and tasidotin, have been progressed to clinical trials for different types of cancer. DL15 has been known to interfere with cellular microtubules and purified tubulin in vitro. However, the molecular mechanism with which the peptide arrests cells in mitosis is poorly understood. This study reports a possible antimitotic mechanism of action of DL15. DL15 inhibited HeLa cell proliferation in a concentration-dependent manner with a half-maximal inhibitory concentration (IC50) of 2.8 ± 0.3 nM, induced mitotic arrest, disrupted cellular microtubules near its IC50 for cell proliferation, and inhibited the re-polymerization of cellular microtubules. By staining the centrosomes of DL15-treated cells with anti-γ tubulin antibodies, the study found a significant reduction in interpolar distances in mitotic HeLa cells, indicating a disruption in the normal assembly dynamics of the microtubules. The study further found that DL15 induced a loss of tension across the kinetochore pairs as indicated by a reduction in interkinetochore distance. In response to this loss of tension, the tension-sensing checkpoint protein BuBR1 accumulated at the kinetochores, promoting mitotic arrest. In vitro, DL15 promoted formation of curved and fragmented polymers of microtubule proteins and inhibited tubulin decay in a manner similar to vinca-site binding agents such as phomopsin A. Together, the data indicate that the mitotic arrest induced by DL15 involves a loss of tension across the kinetochore pairs due to disruption of normal assembly dynamics of microtubules.





Similar content being viewed by others
References
Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell J (2000) Molecular cell biology, 4th edn. WH Freeman & Company, New York
McIntosh JR, Grishchuk EL, West RR (2002) Chromosome-microtubule interactions during mitosis. Annu Rev Cell Dev Biol 18:193–219. doi:10.1146/annurev.cellbio.18.032002.132412
Lopus M, Yenjerla M, Wilson L (2008) Microtubule dynamics. In Wiley Encyclopedia of Chemical Biology. doi:10.1002/9780470048672.wecb338
Avila J (1992) Microtubule functions. Life Sci 50:327–334
Dumontet C, Jordan MA (2010) Microtubule-binding agents: a dynamic field of cancer therapeutics. Nat Rev Drug Discov 9:790–803. doi:10.1038/nrd3253
Hinow P, Rezania V, Lopus M, Jordan MA, Tuszyński JA (2011) Modeling the effects of drug binding on the dynamic instability of microtubules. Phys Biol 8:056004. doi:10.1088/1478-3975/8/5/056004
Pettit GR, Kamano Y, Dufresne C, Cerny RL, Herald CL, Schmidt JM (1989) Isolation and structure of the cytostatic linear depsipeptide dolastatin 15. J Org Chem 54:6005–6006. doi:10.1021/jo00287a003
Hu ZB, Gignac SM, Quentmeier H, Pettit GR, Drexler HG (1993) Effects of dolastatins on human B-lymphocytic leukemia cell lines. Leuk Res 17:333–339. doi:10.1016/0145-2126(93)90020-L
Ebbinghaus S, Rubin E, Hersh E, Cranmer LD, Bonate PL, Fram RJ, Jekunen A, Weitman S, Hammond LA (2005) A phase I study of the dolastatin-15 analogue tasidotin (ILX651) administered intravenously daily for 5 consecutive days every 3 weeks in patients with advanced solid tumors. Clin Cancer Res 11:7807–7816. doi:10.1158/1078-0432.CCR-05-0909
Molinski TF, Dalisay DS, Lievens SL, Saludes JP (2008) Drug development from marine natural products. Nat Rev Drug Discov 8:69–85. doi:10.1038/nrd2487
Mita AC, Hammond LA, Bonate PL et al (2006) Phase I and pharmacokinetic study of tasidotin hydrochloride (ILX651), a third-generation dolastatin-15 analogue, administered weekly for 3 weeks every 28 days in patients with advanced solid tumors. Clin Cancer Res 12:5207–5215. doi:10.1158/1078-0432.CCR-06-0179
Cunningham C, Appleman LJ, Kirvan-Visovatti M et al (2005) Phase I and pharmacokinetic study of the dolastatin-15 analogue tasidotin (ILX651) administered intravenously on days 1, 3, and 5 every 3 weeks in patients with advanced solid tumors. Clin Cancer Res 11:7825–7833. doi:10.1158/1078-0432.CCR-05-0058
Bai R, Covell DG, Taylor GF, Kepler JA, Copeland TD, Nguyen NY, Pettit GR, Hamel E (2004) Direct photoaffinity labeling by dolastatin 10 of the amino-terminal peptide of β-tubulin containing cysteine 12. J Biol Chem 279:30731–30740. doi:10.1074/jbc.M402110200
Mitra A, Sept D (2004) Localization of the antimitotic peptide and depsipeptide binding site on beta-tubulin. Biochemistry 43:13955–13962. doi:10.1021/bi0487387
Cruz-Monserrate Z, Mullaney JT, Harran PG, Pettit GR, Hamel E (2003) Dolastatin 15 binds in the vinca domain of tubulin as demonstrated by Hummel–Dreyer chromatography. Eur J Biochem 270:3822–3828. doi:10.1046/j.1432-1033.2003.03776.x
Kotha S, Kashinath D, Lopus M, Panda D (2009) Synthesis of nano-sized C3-symmetric 2,4,6-triphenyl-l,3,5-s- triazine and 1,3,5 triphenylbenzene derivatives via the trimerization followed by Suzuki–Miyaura cross-coupling or O-alkylation reactions and their biological evaluation. Indian J Chem 48:1766–1770
Lopus M, Panda D (2006) The benzophenanthridine alkaloid sanguinarine perturbs microtubule assembly dynamics through tubulin binding. A possible mechanism for its antiproliferative activity. FEBS J 273:2139–2150. doi:10.1111/j.1742-4658.2006.05227.x
Rathinasamy K, Panda D (2006) Suppression of microtubule dynamics by benomyl decreases tension across kinetochore pairs and induces apoptosis in cancer cells. FEBS J 273:4114–4128. doi:10.1111/j.1742-4658.2006.05413.x
Skoufias DA, Andreassen PR, Lacroix FB, Wilson L, Margolis RL (2001) Mammalian Mad2 and Bub1/BubR1 recognize distinct spindle-attachment and kinetochore-tension checkpoints. Proc Natl Acad Sci USA 98:4492–4497. doi:10.1073/pnas.081076898
Lopus M, Manatschal C, Buey RM, Bjelić S, Miller HP, Steinmetz MO, Wilson L (2012) Cooperative stabilization of microtubule dynamics by EB1 and CLIP-170 involves displacement of stably bound Pi at microtubule ends. Biochemistry 51:3021–3030. doi:10.1021/bi300038t
Zhou J, Yao J, Joshi HC (2002) Attachment and tension in the spindle assembly checkpoint. J Cell Sci 115:3547–3555. doi:10.1242/jcs.00029
Prasad AR, Luduena RF, Horowitz PM (1986) Bis (8-anilinonaphthalene-1-sulfonate) as a probe for tubulin decay. Biochemistry 25:739–742. doi:10.1021/bi00351a035
Uzbekov R, Kireyev I, Prigent C (2002) Centrosome separation: respective role of microtubules and actin filaments. Biol Cell 94:275–288
Nicklas RB, Ward SC (1994) Elements of error correction in mitosis: microtubule capture, release, and tension. J Cell Biol 126:1241–1253. doi:10.1083/jcb.126.5.1241
Rieder CL, Schultz A, Cole R, Sluder G (1994) Anaphase onset in vertebrate somatic cells is controlled by a checkpoint that monitors sister kinetochore attachment to the spindle. J Cell Biol 127:1301–1310. doi:10.1083/jcb.127.5.1301
Zhou J, Panda D, Landen JW, Wilson L, Joshi HC (2002) Minor alteration of microtubule dynamics causes loss of tension across kinetochore pairs and activates the spindle checkpoint. J Biol Chem 277:17200–17208
Waters JC, Mitchison TJ, Rieder CL, Salmon ED (1996) The kinetochore microtubule minus-end disassembly associated with poleward flux produces a force that can do work. Mol Biol Cell 7:1547–1558
Morrow CJ, Tighe A, Johnson VL, Scott MI, Ditchfield C, Taylor SS (2005) Bub1 and Aurora B cooperate to maintain BubR1-mediated inhibition of APC/CCdc20. J Cell Sci 118:3639–3652. doi:10.1242/jcs.02487
Bai R, Friedman SJ, Pettit GR, Hamel E (1992) Dolastatin 15, a potent antimitotic depsipeptide derived from Dolabella auricularia. Interaction with tubulin and effects of cellular microtubules. Biochem Pharmacol 43:2637–2645. doi:10.1016/0006-2952(92)90153-A
Luduena RF, Prasad V, Roach MC, Lacey E (1989) Interaction of phomopsin A with bovine brain tubulin. Arch Biochem Biophys 272:32–38
Sullivan AS, Prasad V, Roach MC, Takahashi M, Iwasaki S, Luduena RF (1990) The interaction of rhizoxin with bovine brain tubulin. Cancer Res 50:4277–4280
Cormier A, Marchand M, Ravelli RB, Knossow M, Gigant B (2008) Structural insight into the inhibition of tubulin by vinca domain peptide ligands. EMBO Rep 9:1101–1106. doi:10.1038/embor.2008.171
Ranaivoson FM, Gigant B, Berritt S, Joullié M, Knossow M (2012) Structural plasticity of tubulin assembly probed by vinca-domain ligands. Acta Crystallogr D Biol Crystallogr 68:927–934. doi:10.1107/S0907444912017143
Acknowledgments
The author thanks Prof. Dulal Panda, Department of Biosciences and Bioengineering, Indian Institute of Technology Bombay, for providing the facilities for carrying out this research and for helpful suggestions, and Silja Joseph, University of California, Santa Barbara, for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lopus, M. Mechanism of mitotic arrest induced by dolastatin 15 involves loss of tension across kinetochore pairs. Mol Cell Biochem 382, 93–102 (2013). https://doi.org/10.1007/s11010-013-1721-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-013-1721-8