Abstract
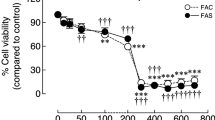
Hepcidin is known to increase intracellular iron through binding to and degrading ferroportin, which is a transmembrane protein that transports iron from the intracellular to the outside. However, it is not clear whether hepcidin has a similar effect on intracellular calcium. Here, we investigated the influence of hepcidin on intracellular calcium in human osteoblasts, with or without high environmental iron concentrations. Our data showed that hepcidin (<100 nmol/L) could increase intracellular calcium, and this effect was more significant when cells were exposed to high environmental iron concentrations. To further explore its underlying mechanisms, we pretreated human osteoblasts with Nimodipine, a L-type calcium channel blocker, and Dantrolene, a ryanodine receptor antagonist to inhibit abnormal calcium release from the sarco-endoplasmic reticulum. These treatments had not resulted in any alteration of intracellular calcium in human osteoblasts. Thus, these findings indicate that the increase of intracellular calcium induced by hepcidin is probably due to calcium release from endoplasmic reticulum, which is triggered by calcium influx.




Similar content being viewed by others
References
Weinberg ED (2006) Iron loading: a risk factor for osteoporosis. Biometals 19:633–635
Yamaguchi M (2010) Role of nutritional zinc in the prevention of osteoporosis. Mol Cell Biochem 338:241–254
Yamasaki K, Hagiwara H (2009) Excess iron inhibits osteoblast metabolism. Toxicol Lett 191:211–215
Liu G, Men P, Kenner GH, Miller SC (2006) Age-associated iron accumulation in bone: implications for postmenopausal osteoporosis and a new target for prevention and treatment by chelation. Biometals 19:245–251
Qu ZH, Zhang XL, Tang TT, Dai KR (2008) Promotion of osteogenesis through beta-catenin signaling by desferrioxamine. Biochem Biophys Res Commun 370:332–337
Valenti L, Varenna M, Fracanzani AL, Rossi V, Fargion S, Sinigaglia L (2009) Association between iron overload and osteoporosis in patients with hereditary hemochromatosis. Ostroporos Int 20:549–555
Tsay J, Yang Z, Ross FP, Cunningham-Rundles S, Lin H, Coleman R, Mayer-Kuckuk P, Doty SB, Grady RW, Giardina PJ, Boskey AL, Vogiatzi MG (2010) Bone loss caused by iron overload in a murine model: importance of oxidative stress. Blood 116:2582–2589
Li GF, Pan YZ, Sirois P, Li K, Xu YJ (2012) Iron homeostasis in osteoporosis and its clinical implications. Osteoporos Int. doi:10.1007/s00198-012-1982-1
Park CH, Valore EV, Waring AJ, Ganz T (2001) Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem 276:7806–7810
Zhang AS, Enns CA (2009) Molecular mechanisms of normal iron homeostasis. Hematol Am Soc Hematol Educ Program 1:207–214
Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J (2004) Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306:2090–2093
De Domenico I, Ward DM, Langelier C, Vaughn MB, Nemeth E, Sundquist WI, Ganz T, Musci G, Kaplan J (2007) The molecular mechanism of hepcidin-mediated ferroportin down-regulation. Mol Biol Cell 18:2569–2578
Rivera S, Nemeth E, Gabayan V, Lopez MA, Farshidi D, Ganz T (2005) Synthetic hepcidin causes rapid dose-dependent hypoferremia and is concentrated in ferroportin-containing organs. Blood 106:2196–2199
Xu Y, Li G, Du B, Zhang P, Xiao L, Sirois P, Li K (2011) Hepcidin increases intracellular Ca2+ of osteoblast hFOB1.19 through L-type Ca2+ channels. Regul Pept 172:58–61
Berridge MJ, Lipp P, Bootman MD (2000) Signal transduction. The calcium entry pas de deux. Science 287:1604–1605
Labelle D, Jumarie C, Moreau R (2007) Capacitative calcium entry and proliferation of human osteoblast-like MG-63 cells. Cell Prolif 40:866–884
Jang HO, Park YS, Lee JH, Seo JB, Koo KI, Jeong SC, Jin SD, Lee YH, Eom HS, Yun I (2007) Effect of extracts from safflower seeds on osteoblast differentiation and intracellular calcium ion concentration in MC3T3-E1 cells. Nat Prod Res 21:787–797
Tsay J, Yang Z, Ross FP, Cunningham-Rundles S, Lin H, Coleman R, Mayer-Kuckuk P, Doty SB, Grady RW, Giardina PJ, Boskey AL, Vogiatzi MG (2010) Bone loss caused by iron overload in a murine model: importance of oxidative stress. Blood 116:2582–2589
Kudo H, Suzuki S, Watanabe A, Kikuchi H, Sassa S, Sakamoto S (2008) Effects of colloidal iron overload on renal and hepatic siderosis and the femur in male rats. Toxicology 246:143–147
Jian J, Pelle E, Huang X (2009) Iron and menopause: does increased iron affect the health of postmenopausal women? Antioxid Redox Signal 11:2939–2943
Guggenbuhl P, Filmon R, Mabilleau G, Basle MF, Chappard D (2008) Iron inhibits hydroxyapatite crystal growth in vitro. Metabolism 57:903–910
Lauckner JE, Hille B, Mackie K (2005) The cannabinoid agonist WIN55,212–2 increases intracellular calcium via CB1 receptor coupling to Gq/11 G proteins. Proc Natl Acad Sci USA 102:19144–19149
Varadi A, Rutter GA (2004) Ca2+-induced Ca2+ release in pancreatic islet beta-cells: critical evaluation of the use of endoplasmic reticulum-targeted “cameleons”. Endocrinology 145:4540–4549
Lemmens R, Larsson O, Berggren PO, Islam MS (2001) Ca2+-induced Ca2+ release from the endoplasmic reticulum amplifies the Ca2+ signal mediated by activation of voltage-gated L-type Ca2+ channels in pancreatic beta-cells. J Biol Chem 276:9971–9977
Graves TK, Hinkle PM (2003) Ca(2+)-induced Ca(2+) release in the pancreatic beta-cell: direct evidence of endoplasmic reticulum Ca(2+) release. Endocrinology 144:3565–3574
Choi KJ, Cho DS, Kim JY, Kim BJ, Lee KM, Kim SH, Kim DK, Kim SH, Park HS (2011) Ca-induced Ca release from internal stores in INS-1 rat insulinoma cells. Korean J Physiol Pharmacol 15:53–59
Acknowledgments
This research is supported by Natural Science Foundation of Jiangsu Province (BK2008165), Programs Foundation of Ministry of Education of China (20103201110020) and Postgraduate Innovation Fund of Jiangsu Province (CX10B-053Z).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, GF., Xu, YJ., He, YF. et al. Effect of hepcidin on intracellular calcium in human osteoblasts. Mol Cell Biochem 366, 169–174 (2012). https://doi.org/10.1007/s11010-012-1294-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-012-1294-y