Abstract
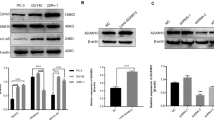
A disintegrin and metalloprotease 17 (ADAM17) is a transmembrane protein that can cleave membrane anchored proteins to release soluble factors and regulate important biological phenomena in cancers. In the present study, we evaluated the effects of ADAM17 on the proliferation and on the cell cycle distribution of human prostate cancer cells. Experiments were also performed to gain insights into the possible mechanism of action of ADAM17. We used over-expression and RNAi strategy to investigate the function of ADAM17 in human prostate cancer cells. Changes in rate of proliferation and cell cycle profile were measured by growth curve, Cell Counting Kit-8 (CCK-8) assay, bromodeoxyuridine (BrdU) incorporation assay and cell cycle analysis. In addition, changes in expression of associated genes and proteins were studied by semiquantitative RT-PCR, western blotting and ELISA analysis. Ectopic over-expression of ADAM17 resulted in increased cell proliferation. We also showed that ADAM17 promoted G1 to S phase transition concomitantly with upregulation of cyclin E, CDK2 and downregulation of p21 and p27 proteins. ADAM17 over-expression cells showed that more TGF-α released to the supernatant and activated the EGFR/PI3K/AKT pathway. Conversely, silencing ADAM17 led to the opposite effect. Both siRNAs knockdown of ADAM17 and blocking the EGFR/PI3K/AKT pathway using specific inhibitor caused downregulation of cyclin E, CDK2, and upregulation of p21 and p27 in prostate cancer cells. Collectively, this study demonstrates that over-expression of ADAM17 might target cyclin E, CDK2, p21, and p27 to promote prostate cancer cell proliferation through activation of the EGFR/PI3K/AKT pathway.






Similar content being viewed by others
References
Moul JW (2003) Population screening for prostate cancer and emerging concepts for young men. Clin Prostate Cancer 2:87–97
D’Amico AV (2003) Therapeutic strategies for localized and locally advanced prostate cancer: combining androgen suppression with definitive local therapy. Rev Urol 5:S40–S46
Brawer MK, Crawford ED, Labrie F, Mendoza-Valdes A, Miller PD, Petrylak DP (2001) Androgen deprivation and other treatments for advanced prostate cancer. Rev Urol 3:S59–S68
Peschon JJ, Slack JL, Reddy P, Stocking KL, Sunnarborg SW, Lee DC, Russell WE, Castner BJ, Johnson RS, Fitzner JN, Boyce RW, Nelson N, Kozlosky CJ, Wolfson MF, Rauch CT, Cerretti DP, Paxton RJ, March CJ, Black RA (1998) An essential role for ectodomain shedding in mammalian development. Science 282:1281–1284
Merlos-Sua Ârez A, Ruiz-Paz S, Baselga J, Arribas J (2001) Metalloprotease-dependent proTGF-a ectodomain shedding in the absence of TACE. J Biol Chem 276:48510–48517
Sunnarborg SW, Hinkle CL, Stevenson M, Russell WE, Raska CS, Peschon JJ, Castner BJ, Gerhart MJ, Paxton RJ, Black RA, Lee DC (2002) Tumor necrosis factor-a converting enzyme (TACE) regulates epidermal growth factor receptor ligand availability. J Biol Chem 277:12838–12845
Blobel CP (2005) ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol 6:32–43
Hart S, Fischer OM, Ullrich A (2004) Cannabinoids induce cancer cell proliferation via tumor necrosis factor alpha-converting enzyme (TACE/ADAM17)-mediated transactivation of the epidermal growth factor receptor. Cancer Res 64:1943–1950
Kodama T, Ikeda E, Okada A, Ohtsuka T, Shimoda M, Shiomi T, Yoshida K, Nakada M, Ohuchi E, Okada Y (2004) ADAM12 is selectively overexpressed in human glioblastomas and is associated with glioblastoma cell proliferation and shedding of heparin-binding epidermal growth factor. Am J Pathol 165:1743–1753
Blanchot-Jossic F, Jarry A, Masson D, Bach-Ngohou K, Paineau J, Denis MG, Laboisse CL, Mosnier JF (2005) Up-regulated expression of ADAM17 in human colon carcinoma: co-expression with EGFR in neoplastic and endothelial cells. J Pathol 207:156–163
Ringel J, Jesnowski R, Moniaux N, Luttges J, Choudhury A, Batra SK, Kloppel G, Lohr M (2006) Aberrant expression of a disintegrin and metalloproteinase 17/tumor necrosis factor-alpha converting enzyme increases the malignant potential in human pancreatic ductal adenocarcinoma. Cancer Res 66:9045–9053
Kenny PA, Bissell MJ (2007) Targeting TACE-dependent EGFR ligand shedding in breast cancer. J Clin Invest 117:337–345
Ruhe JE, Streit S, Hart S, Ullrich A (2006) EGFR signaling leads to downregulation of PTP-LAR via TACE-mediated proteolytic processing. Cell Signal 18:1515–1527
Lee DC, Sunnarborg SW, Hinkle CL, Myers TJ, Stevenson MY, Russell WE, Castner BJ, Gerhart MJ, Paxton RJ, Black RA, Chang A, Jackson LF (2003) TACE/ADAM17 processing of EGFR ligands indicates a role as a physiological convertase. Ann N Y Acad Sci 995:22–38
Kenny PA (2007) Tackling EGFR signaling with TACE antagonists: a rational target for metalloprotease inhibitors in cancer. Expert Opin Ther Targets 11:1287–1298
Borrell-Pages M, Rojo F, Albanell J, Baselga J, Arribas J (2003) TACE is required for the activation of the EGFR by TGF-alpha in tumors. EMBO 22:1114–1124
Ebi M, Kataoka H, Shimura T, Kubota E, Hirata Y, Mizushima T, Mizoshita T, Tanaka M, Mabuchi M, Tsukamoto H, Tanida S, Kamiya T, Higashiyama S, Joh T (2010) TGFbeta induces proHB-EGF shedding and EGFR transactivation through ADAM activation in gastric cancer cells. Biochem Biophys Res Commun 402:449–454
DeHaan AM, Wolters NM, Keller ET, Ignatoski KM (2009) EGFR ligand switch in late stage prostate cancer contributes to changes in cell signaling and bone remodeling. Prostate 69:528–537
Liu XH, Wiley HS, Miekle AW (1993) Androgens regulate proliferation of human prostate cancer cells in culture by increasing transforming growth factor-α and epidermal growth factor/TGF-α receptor. J Clin Endocrinol Metab 77:1472–1478
Glynne Jones E, Goddard L, Harper ME (1996) Comparative analysis of mRNA and protein expression for epidermal growth factor receptor and ligands relative to the proliferative index in human prostate tissue. Hum Pathol 27:688–694
Zhau HY, Chang SM, Chen BQ (1996) Androgen-repressed phenotype in prostate cancer. Proc Natl Acad Sci USA 93:15152–15157
Segrelles C, Moral M, Lara MF, Ruiz S, Santos M, Leis H, Garcia-Escudero R, Martinez-Cruz AB, Martinez-Palacio J, Hernandez P, Ballestin C, Paramio JM (2006) Molecular determinants of Akt-induced keratinocyte transformation. Oncogene 25:1174–1185
Zheng X, Jiang F, Katakowski M, Zhang ZG, Lu QE, Chopp M (2009) ADAM17 promotes breast cancer cell malignant phenotype through EGFR-PI3K-AKT activation. Cancer Biol Ther 8:1045–1054
He YY, Council SE, Feng L, Chignell CF (2008) UVA-induced cell cycle progression is mediated by a disintegrin and metalloprotease/epidermal growth factor receptor/AKT/Cyclin D1 pathways in keratinocytes. Cancer Res 68:3752–3758
Katakowski M, Jiang F, Zheng X, Gutierrez JA, Szalad A, Chopp M (2009) Tumorigenicity of cortical astrocyte cell line induced by the protease ADAM17. Cancer Sci 100:1597–1604
Malumbres M, Barbacid M (2009) Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer 9:153–166
Horiguchi-Yamada J, Yamada H, Nakada S, Ochi K, Nemoto T (1994) Changes of G1 cyclins, cdk2, and cyclin A during the differentiation of HL60 cells induced by TPA. Mol Cell Biochem 132:31–37
He G, Kuang J, Huang Z, Koomen J, Kobayashi R, Khokhar AR, Siddik ZH (2006) Upregulation of p27 and its inhibition of CDK2/cyclin E activity following DNA damage by a novel platinum agent are dependent on the expression of p21. Br J Cancer 95:1514–1524
Dev K, Frank CL, Michael B, Jöerg R, Nicolas M, Ming-Fong L, Surinder KB (2003) Expression of ADAMs (a disintegrin and metalloproteases) and TIMP-3 (tissue inhibitor of metalloproteinase-3) in human prostatic adenocarcinomas. Int J Oncol 23:1365–1371
Wang HX, Wang HM, Lin HY, Yang Q, Zhang H, Tsang BK, Zhu C (2006) Proteasome subunit LMP2 is required for matrix metalloproteinase-2 and -9 expression and activities in human invasive extravillous trophoblast cell line. J Cell Physiol 206:616–623
Shapiro GI (2006) Cyclin-dependent kinase pathways as targets for cancer treatment. J Clin Oncol 24:1770–1783
Lee YM, Sicinski P (2006) Targeting cyclins and cyclin-dependent kinases in cancer: lessons from mice, hopes for therapeutic applications in human. Cell Cycle 5:2110–2114
Obaya AJ, Sedivy JM (2002) Regulation of cyclin-Cdk activity in mammalian cells. Cell Mol Life Sci 59:126–142
Morgan DO (1997) Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol 13:261–291
Cai K, Dynlacht BD (1998) Activity and nature of p21(WAF1) complexes during the cell cycle. Proc Natl Acad Sci USA 95:12254–12259
Ogryzko VV, Wong P, Howard BH (1997) WAF1 retards S-phase progression primarily by inhibition of cyclin-dependent kinases. Mol Cell Biol 17:4877–4882
Egilmez R, Elagoz S, Kanik EA (2001) Cdk1/P34Cdc2 and P21waf expression in colorectal adenomas and carcinomas. J Exp Clin Cancer Res 20:549–552
Stein JP, Ginsberg DA, Grossfeld GD, Chatterjee SJ, Esrig D, Dickinson MG, Groshen S, Taylor CR, Jones PA, Skinner DG, Cote RJ (1998) Effect of p21WAF1/CIP1 expression on tumor progression in bladder cancer. J Natl Cancer Inst 90:1072–1079
Gschwind A, Hart S, Fischer OM, Ullrich A (2003) TACE cleavage of proamphiregulin regulates GPCR-induced proliferation and motility of cancer cells. EMBO 22:2411–2421
Seger R, Krebs EG (1995) The MAPK signaling cascade. FASEB J 9:726–735
Bokemeyer D, Sorokin A, Dunn MJ (1996) Multiple intracellular MAP kinase signaling cascades. Kidney Int 49:1187–1198
Arribas J, Bech-Serra JJ, Santiago-Josefat B (2006) ADAMs, cell migration and cancer. Cancer Metastasis Rev 25:57–68
Acknowledgments
This research is funded by National Natural Science Foundation of China (No. 30772173), Natural Science Foundation of Heilongjiang Province, China (No. D2007-56) and Innovation Fund for Excellent Graduates of Harbin Medical University (No. HCXS2010001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Ping Lin, Xicai Sun, and Tian Feng contributed equally to this study.
Rights and permissions
About this article
Cite this article
Lin, P., Sun, X., Feng, T. et al. ADAM17 regulates prostate cancer cell proliferation through mediating cell cycle progression by EGFR/PI3K/AKT pathway. Mol Cell Biochem 359, 235–243 (2012). https://doi.org/10.1007/s11010-011-1018-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-011-1018-8