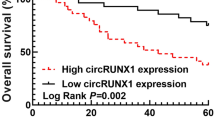
Abstract
High-mobility group A1 (HMGA1) is a non-histone chromatin protein that has the ability to regulate the transcriptional activity of many genes. Overexpression of HMGA1 is associated with malignant cellular behavior in a range of human cancers but the underlying mechanism is largely unknown. Here we showed that in a cohort of non-small cell lung cancer (NSCLC) tumors, HMGA1 overexpression was immediately associated with enhanced expression of an oncogenic miRNA, namely, miR-222. Chromatin immunoprecipitation (CHIP) assay revealed that HMGA1 directly binds to the proximal promoter of miR-222 in NSCLC cells. We further showed that HMGA1 silencing reduced miR-222 transcriptional activity, whereas forced HMGA1 expression increased it, indicating that miR-222 is directly regulated by HMGA1. Based on in silico prediction, one of the putative targets of miR-222 is phosphatase 2A subunit B (PPP2R2A) which inhibits Akt phosphorylation (p-Akt). We demonstrated that miR-222 inhibited protein expression of PPP2R2A in NSCLC cells by directly interacting with its 3′-UTR region, leading to an obvious increase of p-Akt. HMGA1 silencing augmented PPP2R2A protein expression and inhibited Akt signaling, resulting in significantly retarded cell growth response to IGF-I. These results suggested that HMGA1 is a positive regulator of miR-222, and HMGA1 overexpression might contribute to dysregulation of Akt signaling in NSCLC.




Similar content being viewed by others
References
Akaboshi S, Watanabe S, Hino Y, Sekita Y, Xi Y, Araki K, Yamamura K, Oshima M, Ito T, Baba H, Nakao M (2009) HMGA1 is induced by Wnt/beta-catenin pathway and maintains cell proliferation in gastric cancer. Am J Pathol 175:1675–1685
Hillion J, Wood LJ, Mukherjee M, Bhattacharya R, Di Cello F, Kowalski J, Elbahloul O, Segal J, Poirier J, Rudin CM, Dhara S, Belton A, Joseph B, Zucker S, Resar LM (2009) Upregulation of MMP-2 by HMGA1 promotes transformation in undifferentiated, large-cell lung cancer. Mol Cancer Res 7:1803–1812
Treff NR, Dement GA, Adair JE, Britt RL, Nie R, Shima JE, Taylor WE, Reeves R (2004) Human KIT ligand promoter is positively regulated by HMGA1 in breast and ovarian cancer cells. Oncogene 23:8557–8562
Flohr AM, Rogalla P, Bonk U, Puettmann B, Buerger H, Gohla G, Packeisen J, Wosniok W, Loeschke S, Bullerdiek J (2003) High mobility group protein HMGA1 expression in breast cancer reveals a positive correlation with tumour grade. Histol Histopathol 18:999–1004
Pierantoni GM, Rinaldo C, Esposito F, Mottolese M, Soddu S, Fusco A (2006) High Mobility Group A1 (HMGA1) proteins interact with p53 and inhibit its apoptotic activity. Cell Death Differ 13:1554–1563
Pierantoni GM, Rinaldo C, Mottolese M, Di Benedetto A, Esposito F, Soddu S, Fusco A (2007) High-mobility group A1 inhibits p53 by cytoplasmic relocalization of its proapoptotic activator HIPK2. J Clin Invest 117:693–702
Wei JJ, Wu X, Peng Y, Shi G, Olca B, Yang X, Daniels G, Osman I, Ouyang J, Hernando E, Pellicer A, Rhim JS, Melamed J, Lee P (2011) Regulation of HMGA1 expression by microRNA-296 affects prostate cancer growth and invasion. Clin Cancer Res 17:1297–1305
van der Zee JA, ten Hagen TL, Hop WC, van Dekken H, Dicheva BM, Seynhaeve AL, Koning GA, Eggermont AM, van Eijck CH (2011) Differential expression and prognostic value of HMGA1 in pancreatic head and periampullary cancer. Eur J Cancer 46:3393–3399
Frasca F, Rustighi A, Malaguarnera R, Altamura S, Vigneri P, Del Sal G, Giancotti V, Pezzino V, Vigneri R, Manfioletti G (2006) HMGA1 inhibits the function of p53 family members in thyroid cancer cells. Cancer Res 66:2980–2989
Sarhadi VK, Wikman H, Salmenkivi K, Kuosma E, Sioris T, Salo J, Karjalainen A, Knuutila S, Anttila S (2006) Increased expression of high mobility group A proteins in lung cancer. J Pathol 209:206–212
Chau KY, Keane-Myers AM, Fedele M, Ikeda Y, Creusot RJ, Menozzi L, Cousins DJ, Manfioletti G, Feigenbaum L, Fusco A, Ono SJ (2005) IFN-gamma gene expression is controlled by the architectural transcription factor HMGA1. Int Immunol 17:297–306
Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S, Cordon-Cardo C, Pelletier J, Lowe SW (2004) Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature 428:332–337
Kolb S, Fritsch R, Saur D, Reichert M, Schmid RM, Schneider G (2007) HMGA1 controls transcription of insulin receptor to regulate cyclin D1 translation in pancreatic cancer cells. Cancer Res 67:4679–4686
Wang XC, Du LQ, Tian LL, Wu HL, Jiang XY, Zhang H, Li DG, Wang YY, Wu HY, She Y, Liu QF, Fan FY, Meng AM (2011) Expression and function of miRNA in postoperative radiotherapy sensitive and resistant patients of non-small cell lung cancer. Lung Cancer 72(1):92–99
Liau SS, Jazag A, Ito K, Whang EE (2007) Overexpression of HMGA1 promotes anoikis resistance and constitutive Akt activation in pancreatic adenocarcinoma cells. Br J Cancer 96:993–1000
De Martino I, Visone R, Fedele M, Petrocca F, Palmieri D, Martinez Hoyos J, Forzati F, Croce CM, Fusco A (2009) Regulation of microRNA expression by HMGA1 proteins. Oncogene 28:1432–1442
Shin S, Cha HJ, Lee EM, Lee SJ, Seo SK, Jin HO, Park IC, Jin YW, An S (2009) Alteration of miRNA profiles by ionizing radiation in A549 human non-small cell lung cancer cells. Int J Oncol 35:81–86
Keller A, Leidinger P, Gislefoss R, Haugen A, Langseth H, Staehler P, Lenhof HP, Meese E (2011) Stable serum miRNA profiles as potential tool for non-invasive lung cancer diagnosis. RNA Biol 8(3)
Shah AA, Leidinger P, Blin N, Meese E (2010) miRNA: small molecules as potential novel biomarkers in cancer. Curr Med Chem 17:4427–4432
Hulf T, Sibbritt T, Wiklund ED, Bert S, Strbenac D, Statham AL, Robinson MD, Clark SJ (2011) Discovery pipeline for epigenetically deregulated miRNAs in cancer: integration of primary miRNA transcription. BMC Genomics 12:54
O’Hara AJ, Vahrson W, Dittmer DP (2008) Gene alteration and precursor and mature microRNA transcription changes contribute to the miRNA signature of primary effusion lymphoma. Blood 111:2347–2353
Parizotto EA, Dunoyer P, Rahm N, Himber C, Voinnet O (2004) In vivo investigation of the transcription, processing, endonucleolytic activity, and functional relevance of the spatial distribution of a plant miRNA. Genes Dev 18:2237–2242
Zhang J, Han L, Ge Y, Zhou X, Zhang A, Zhang C, Zhong Y, You Y, Pu P, Kang C (2010) miR-221/222 promote malignant progression of glioma through activation of the Akt pathway. Int J Oncol 36:913–920
Liau SS, Jazag A, Whang EE (2006) HMGA1 is a determinant of cellular invasiveness and in vivo metastatic potential in pancreatic adenocarcinoma. Cancer Res 66:11613–11622
Zhang C, Zhang J, Zhang A, Wang Y, Han L, You Y, Pu P, Kang C (2010) PUMA is a novel target of miR-221/222 in human epithelial cancers. Int J Oncol 37:1621–1626
Dai R, Li J, Liu Y, Yan D, Chen S, Duan C, Liu X, He T, Li H (2010) miR-221/222 suppression protects against endoplasmic reticulum stress-induced apoptosis via p27(Kip1)- and MEK/ERK-mediated cell cycle regulation. Biol Chem 391:791–801
Zhang C, Kang C, You Y, Pu P, Yang W, Zhao P, Wang G, Zhang A, Jia Z, Han L, Jiang H (2009) Co-suppression of miR-221/222 cluster suppresses human glioma cell growth by targeting p27kip1 in vitro and in vivo. Int J Oncol 34:1653–1660
Di Leva G, Gasparini P, Piovan C, Ngankeu A, Garofalo M, Taccioli C, Iorio MV, Li M, Volinia S, Alder H, Nakamura T, Nuovo G, Liu Y, Nephew KP, Croce CM (2010) MicroRNA cluster 221–222 and estrogen receptor alpha interactions in breast cancer. J Natl Cancer Inst 102:706–721
Manning BD (2010) Insulin signaling: inositol phosphates get into the Akt. Cell 143:861–863
Kuo YC, Huang KY, Yang CH, Yang YS, Lee WY, Chiang CW (2008) Regulation of phosphorylation of Thr-308 of Akt, cell proliferation, and survival by the B55alpha regulatory subunit targeting of the protein phosphatase 2A holoenzyme to Akt. J Biol Chem 283:1882–1892
Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K, Hatanaka H, Bando M, Ohno S, Ishikawa Y, Aburatani H, Niki T, Sohara Y, Sugiyama Y, Mano H (2007) Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 448:561–566
Restuccia DF, Hemmings BA (2009) Cell signaling. Blocking Akt-ivity. Science 325:1083–1084
Acknowledgments
This work was supported by Eleven Fifth Key Research grant from the Ministry of Science and Technology (No. 2008ZX10001-008), the National Science Foundation of China (30700467), and the Key Project of Chinese National Programs (2008ZX10003-010).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Y., Ma, T., Yang, S. et al. High-mobility group A1 proteins enhance the expression of the oncogenic miR-222 in lung cancer cells. Mol Cell Biochem 357, 363–371 (2011). https://doi.org/10.1007/s11010-011-0907-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-011-0907-1