Abstract
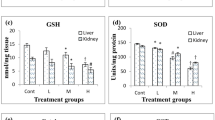
Oxidative stress, a pervasive condition induced by stress has been implicated and recognized to be a prominent feature of various pathological states including cancer and their progression. The present study sought to validate the effectiveness of chronic unpredictable stress (CUS) on hepatic and renal toxicity in terms of alterations of various in vivo biochemical parameters, oxidative stress markers and the extent of DNA damage in Swiss albino mice. Animals were randomized into different groups based on their exposure to CUS alone, 7,12-dimethylbenz (a) anthracene (DMBA) alone (topical), DMBA-12-O-tetradecanoylphorbol-13-acetate (TPA) (topical), and exposure to CUS prior to DMBA or DMBA-TPA treatment, and sacrificed after 16 weeks of treatment. Prior exposure to CUS increased the pro-oxidant effect of carcinogen as depicted by significantly compromised levels of antioxidants; superoxide dismutase, catalase, glutathione-S-transferase, glutathione reductase, reduced glutathione in hepatic and renal tissues accompanied by a significant elevation of thiobarbituric acid reactive species (TBARS) as compared to DMBA alone or DMBA-TPA treatments. Loss of structural integrity at the cellular level due to stress-induced oxidative damage was demonstrated by significant increases in the hepatic levels of intracellular marker enzymes such as glutamate oxaloacetate transaminase, glutamate pyruvate transaminase and alkaline phosphatase, and significantly reduced levels of uric acid in kidney tissues. The results of DNA damage studies further positively correlated with all the above biochemical measurements. Thus, exposure to physical or psychological stress may significantly enhance the hepatotoxic and nephrotoxic potential of carcinogens through enhanced oxidative stress even if the treatment is topical.





Similar content being viewed by others
References
Antoni MH, Lutgendorf SK, Cole SW et al (2006) The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer 6:240–248
Liu J, Mori A (1999) Stress, aging and oxidative damage. Neurochem Res 24:1479–1497
Toyokuni S, Sagripanti JL (1996) Association between 8-hydroxy-20-deoxyguanosine formation and DNA strand breaks mediated by copper and iron. Free Radic Biol Med 20:859–864
Toyokuni S, Mori T, Hiai H, Dizdaroglu M (1995) Treatment of Wistar rats with a renal carcinogen, ferric nitrilotriacetate, causes DNA-protein cross-linking between thymine and tyrosine in their renal chromatin. Int J Cancer 62:309–313
Nakabeppu Y (2001) Regulation of intracellular localization of human MTH1, OGG1, and MYH proteins for repair of oxidative DNA damage. Prog Nucleic Acid Res Mol Biol 68:75–94
Wijnhoven SWP, Kool HJM, Mullenders LHF et al (2001) DMBA-induced toxic and mutagenic response vary dramatically between NER-deficient Xpa, Xpc and Csb mice. Carcinogenesis 22:1099–1106
Buters J, Quintanilla-Martinez L, Schober W et al (2003) CYP1B1 determines susceptibility to low doses of 7,12-dimethylbenz (a) anthracene induced ovarian cancers in mice: correlation of CYP1B1-mediated DNA adducts with carcinogenicity. Carcinogenesis 24:327–334
Al-Attar AM (1998) Physiological studies on the effect of fish oil on liver tumour induced by DMBA in the Egyptian toad. PhD thesis, Alexandria University, Egypt
Rose ML, Rivera CA, Bradford BU et al (1999) Kupffer cell oxidant production is central to the mechanism of peroxisome proliferators. Carcinogenesis 20:27–33
Rusyn I, Bradham CA, Cohn L et al (1999) Corn oil rapidly activates nuclear factor- kappaB in hepatic Kupffer cells by oxidant dependent mechanisms. Carcinogenesis 20:2095–2100
Parke DV (1994) The cytochromes P450 and mechanisms of chemical carcinogenesis. Environ Health Perspect 102:852–853
Parke DV, Sapota A (1996) Chemical toxicity and reactive oxygen species. Int J Occ Med Environ Health 9:331–340
Okamoto K, Toyokuni S, Uchida K et al (1994) Formation of 8-hydroxy-2-deoxyguanosine and 4-hydroxy-2-nonenal-modified proteins in human renal-cell carcinoma. Int J Cancer 58:825–829
Toyokuni S, Luo XP, Tanaka T et al (1997) Induction of a wide range of C(2–12) aldehydes and C(7–12) acyloins in the kidney of Wistar rats after treatment with a renal carcinogen, ferric nitrilotriacetate. Free Radic Biol Med 22:1019–1027
Toyokuni S, Mori T, Dizdaroglu M (1994) DNA base modifications in renal chromatin of Wistar rats treated with a renal carcinogen, ferric nitrilotriacetate. Int J Cancer 57:123–128
Katz RJ, Roth KA, Carroll BJ (1981) Acute and chronic effects on open field activity in the rat: implications for a model of depression. Neurosci Biobehav Revs 5:247–251
Willner P, Towell A, Sampson D et al (1987) Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology 93:358–364
Marklund S, Marklund G (1974) The involvement of the superoxide anion radical in the auto oxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
Claiborne A (1985) Catalase activity. In: Green Wald RA (ed) CRC handbook of methods for oxygen radical research. CRC Press, Boca Raton, pp 283–284
Habig WH, Pabst MJ, Jacoby WH (1974) Glutathione-s-transferases: the first step in mercapturic acid formation. J Biol Chem 249:7130–7139
Carlberg I, Mannervik B (1975) Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J Biol Chem 250:5475–5480
Jollow DJ, Mitchell JR, Zampaglione N, Gillete JR (1974) Bromobenzene induced liver necrosis: protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic intermediate. Pharmacology 11:151–169
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
Shah SV, Kempson SA, Northrup TE, Dousa TP (1979) Renal adaptation to low phosphate diet in rats. J Clin Invest 64:955–966
Lowry OH, Rosenberg NJ, Farr AL, Randall RJ (1951) Protein measurement with folin phenol reagent. J Biol Chem 193:265–275
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191
Muqbil I, Azmi AS, Banu N (2006) Prior exposure to restraint stress enhances 7,12-dimethylbenz (a) anthracene (DMBA) induced DNA damage in rats. FEBS Lett 580:3995–3999
Harada LT, Tamada K, Abe K, Nomoto K (1997) Repeated restraint stress impairs the antitumor T cell response through its suppressive effect on Th1-type CD4+ T cells. Anticancer Res 17:4259–4268
Boyd SC, Sasame HA, Boyd MR (1981) Effect of cold restraint stress on gastric and hepatic glutathione: a potential determinant of response to chemical carcinogens. Physiol Behav 27:377–379
Liu J, Wang X, Mori A (1994) Immobilization stress induced antioxidant defence changes in rat plasma: effect of treatment with reduced glutathione. Int J Biochem 26:511–517
Guerin MR (1978) Energy sources of polycyclic aromatic hydrocarbons. In: Gelboin HV, TS’o POP (eds) Polycyclic hydrocarbons and cancer: chemistry, molecular biology and environment. Academic press, New York, pp 1–42
Das UN (2002) A radical approach to cancer. Med Sci Monit 8:79–92
Tisdale MJ, Mahmoud MB (1983) Activities of free radical metabolizing enzymes in tumours. Br J Cancer 47:809–812
Locigno R, Castronovo V (2001) Reduced glutathione system; role of cancer development, prevention and treatment (Review). Int J Oncol 19:221–236
Adachi S, Kawamura K, Takemoto K (1993) Oxidative damage of nuclear DNA in liver of rats exposed to psychological stress. Cancer Res 53:4153–4155
Hoeijimakers JH (2001) Genome maintenance mechanisms for preventing cancer. Nature 411:366–374
Muqbil I, Banu N (2006) Enhancement of pro oxidant effect of dimethylbenz (a) anthracene (DMBA) in rats by pre exposure to restraint stress. Cancer Lett 240:213–220
Quinlan GJ, Gutteridge MC (1987) Oxygen radical damage to DNA by rifamycin SV and copper ions. Biochem Pharmacol 36:3629–3633
Frenkel K, Wei L, Wei H (1995) 7,12-Dimethylbenz (a) anthracene induces oxidative DNA modification in vivo. Free Radic Biol Med 19:373–380
Acknowledgments
Thanks are due to University Grants Commission (UGC), New Delhi for financial support to the authors (Nida Suhail, Nayeem Bilal, and Shirin Hasan) in the form of a scholarship. The authors are grateful to UGC-SAP, DST-FIST, and Aligarh Muslim University for the facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suhail, N., Bilal, N., Hasan, S. et al. Chronic unpredictable stress exacerbates 7,12-dimethylbenz (a) anthracene induced hepatotoxicity and nephrotoxicity in Swiss albino mice. Mol Cell Biochem 355, 117–126 (2011). https://doi.org/10.1007/s11010-011-0845-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-011-0845-y