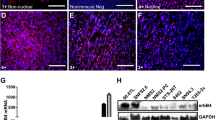
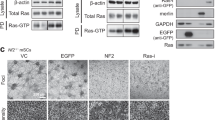
Abstract
Plexiform neurofibromas commonly found in patients with Neurofibromatosis type I (NF1) have a 5% risk of being transformed into malignant peripheral nerve sheath tumors (MPNST). Germline mutations in the NF1 gene coding for neurofibromin, which is a Ras GTPase activating protein (RasGAP) and a negative regulator of Ras, result in an upregulation of the Ras pathway. We established a direct connection between neurofibromin deficiency and downstream effectors of Ras in cell lines from MPNST patients by demonstrating that knockdown of NF1 expression using siRNA in a NF1 wild type MPNST cell line, STS-26T, activates the Ras/ERK1,2 pathway and increases AP-1 binding and activity. We believe this is the first time the transactivation of AP-1 has been linked directly to neurofibromin deficiency in a disease relevant MPNST cell line. Previously, we have shown that N-Ras is constitutively activated in cell lines derived from independent MPNSTs from NF1 patients. We therefore sought to analyze the role of the N-Ras pathway in deregulating AP-1 transcriptional activity. We show that STS-26T clones conditionally expressing oncogenic N-Ras show increased phosphorylated ERK1,2 and phosphorylated JNK expression concomitant with increased AP-1 activity. MAP kinase pathways (ERK1,2 and JNK) were further examined in ST88-14, a neurofibromin-deficient MPNST cell line. The basal activity of ERK1,2 but not JNK was found to increase AP-1 activity. These experiments further confirmed the link between the loss of neurofibromin and increased activity of Ras/MAP kinase pathways and the activation of downstream transcriptional mechanisms in MPNSTs from NF1 patients.






Similar content being viewed by others
References
Levy P, Vidaud D, Leroy K, Laurendeau I, Wechsler J, Bolasco G, Parfait B, Wolkenstein P, Vidaud M, Bieche I (2004) Molecular profiling of malignant peripheral nerve sheath tumors associated with neurofibromatosis type 1, based on large-scale real-time RT-PCR. Mol Cancer 3:20
DeClue JE, Papageorge AG, Fletcher JA, Diehl SR, Ratner N, Vass WC, Lowy DR (1992) Abnormal regulation of mammalian p21ras contributes to malignant tumor growth in von Recklinghausen (type 1) neurofibromatosis. Cell 69:265–273
Basu TN, Gutmann DH, Fletcher JA, Glover TW, Collins FS, Downward J (1992) Aberrant regulation of ras proteins in malignant tumour cells from type 1 neurofibromatosis patients. Nature 356:713–715
Xu GF, O’Connell P, Viskochil D, Cawthon R, Robertson M, Culver M, Dunn D, Stevens J, Gesteland R, White R et al (1990) The neurofibromatosis type 1 gene encodes a protein related to GAP. Cell 62:599–608
Gille H, Downward J (1999) Multiple ras effector pathways contribute to G(1) cell cycle progression. J Biol Chem 274:22033–22040
Pruitt K, Der CJ (2001) Ras and Rho regulation of the cell cycle and oncogenesis. Cancer Lett 171:1–10
Saxena N, Lahiri SS, Hambarde S, Tripathi RP (2008) RAS: target for cancer therapy. Cancer Invest 26:948–955
Liu JJ, Chao JR, Jiang MC, Ng SY, Yen JJ, Yang-Yen HF (1995) Ras transformation results in an elevated level of cyclin D1 and acceleration of G1 progression in NIH 3T3 cells. Mol Cell Biol 15:3654–3663
Johnson R, Spiegelman B, Hanahan D, Wisdom R (1996) Cellular transformation and malignancy induced by ras require c-jun. Mol Cell Biol 16:4504–4511
Smeal T, Binetruy B, Mercola DA, Birrer M, Karin M (1991) Oncogenic and transcriptional cooperation with Ha-Ras requires phosphorylation of c-Jun on serines 63 and 73. Nature 354:494–496
Behrens A, Jochum W, Sibilia M, Wagner EF (2000) Oncogenic transformation by ras and fos is mediated by c-Jun N-terminal phosphorylation. Oncogene 19:2657–2663
Brown PH, Alani R, Preis LH, Szabo E, Birrer MJ (1993) Suppression of oncogene-induced transformation by a deletion mutant of c-jun. Oncogene 8:877–886
Young MR, Li JJ, Rincon M, Flavell RA, Sathyanarayana BK, Hunziker R, Colburn N (1999) Transgenic mice demonstrate AP-1 (activator protein-1) transactivation is required for tumor promotion. Proc Natl Acad Sci USA 96:9827–9832
Shaulian E, Karin M (2002) AP-1 as a regulator of cell life and death. Nat Cell Biol 4:E131–E136
Shen Q, Uray IP, Li Y, Krisko TI, Strecker TE, Kim HT, Brown PH (2008) The AP-1 transcription factor regulates breast cancer cell growth via cyclins and E2F factors. Oncogene 27:366–377
Bahassiel M, Karyala S, Tomlinson CR, Sartor MA, Medvedovic M, Hennigan RF (2004) Critical regulation of genes for tumor cell migration by AP-1. Clin Exp Metastasis 21:293–304
Kim S, Choi JH, Kim JB, Nam SJ, Yang JH, Kim JH, Lee JE (2008) Berberine suppresses TNF-alpha-induced MMP-9 and cell invasion through inhibition of AP-1 activity in MDA-MB-231 human breast cancer cells. Molecules 13:2975–2985
Tan TW, Yang WH, Lin YT, Hsu SF, Li TM, Kao ST, Chen WC, Fong YC, Tang CH (2009) Cyr61 increases migration and MMP-13 expression via alphavbeta3 integrin, FAK, ERK and AP-1-dependent pathway in human chondrosarcoma cells. Carcinogenesis 30:258–268
Ye FC, Blackbourn DJ, Mengel M, Xie JP, Qian LW, Greene W, Yeh IT, Graham D, Gao SJ (2007) Kaposi’s sarcoma-associated herpesvirus promotes angiogenesis by inducing angiopoietin-2 expression via AP-1 and Ets1. J Virol 81:3980–3991
Matthews CP, Colburn NH, Young MR (2007) AP-1 a target for cancer prevention. Curr Cancer Drug Targets 7:317–324
Eferl R, Wagner EF (2003) AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer 3:859–868
Shaulian E, Karin M (2001) AP-1 in cell proliferation and survival. Oncogene 20:2390–2400
Zhu Y, Liao H, Wang N, Ma KS, Verna LK, Shyy JY, Chien S, Stemerman MB (2001) LDL-activated p38 in endothelial cells is mediated by Ras. Arterioscler Thromb Vasc Biol 21:1159–1164
Gille H, Sharrocks AD, Shaw PE (1992) Phosphorylation of transcription factor p62TCF by MAP kinase stimulates ternary complex formation at c-fos promoter. Nature 358:414–417
Clarke N, Arenzana N, Hai T, Minden A, Prywes R (1998) Epidermal growth factor induction of the c-jun promoter by a Rac pathway. Mol Cell Biol 18:1065–1073
Derijard B, Hibi M, Wu IH, Barrett T, Su B, Deng T, Karin M, Davis RJ (1994) JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell 76:1025–1037
Mattingly RR, Kraniak JM, Dilworth JT, Mathieu P, Bealmear B, Nowak JE, Benjamins JA, Tainsky MA, Reiners JJ Jr (2006) The mitogen-activated protein kinase/extracellular signal-regulated kinase kinase inhibitor PD184352 (CI-1040) selectively induces apoptosis in malignant schwannoma cell lines. J Pharmacol Exp Ther 316:456–465
Wojtkowiak JW, Fouad F, LaLonde DT, Kleinman MD, Gibbs RA, Reiners JJ Jr, Borch RF, Mattingly RR (2008) Induction of apoptosis in neurofibromatosis type 1 malignant peripheral nerve sheath tumor cell lines by a combination of novel farnesyl transferase inhibitors and lovastatin. J Pharmacol Exp Ther 326:1–11
Barkan B, Starinsky S, Friedman E, Stein R, Kloog Y (2006) The Ras inhibitor farnesylthiosalicylic acid as a potential therapy for neurofibromatosis type 1. Clin Cancer Res 12:5533–5542
Yan N, Ricca C, Fletcher J, Glover T, Seizinger BR, Manne V (1995) Farnesyltransferase inhibitors block the neurofibromatosis type I (NF1) malignant phenotype. Cancer Res 55:3569–3575
Dilworth JT, Wojtkowiak JW, Mathieu P, Tainsky MA, Reiners JJ Jr, Mattingly RR, Hancock CN (2008) Suppression of proliferation of two independent NF1 malignant peripheral nerve sheath tumor cell lines by the pan-ErbB inhibitor CI-1033. Cancer Biol Ther 7:1938–1946
Reynolds JE, Fletcher JA, Lytle CH, Nie L, Morton CC, Diehl SR (1992) Molecular characterization of a 17q11.2 translocation in a malignant schwannoma cell line. Hum Genet 90:450–456
Mattingly RR, Felczak A, Chen CC, McCabe MJ Jr, Rosenspire AJ (2001) Low concentrations of inorganic mercury inhibit Ras activation during T cell receptor-mediated signal transduction. Toxicol Appl Pharmacol 176:162–168
Dignam JD, Lebovitz RM, Roeder RG (1983) Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res 11:1475–1489
Cichowski K, Santiago S, Jardim M, Johnson BW, Jacks T (2003) Dynamic regulation of the Ras pathway via proteolysis of the NF1 tumor suppressor. Genes Dev 17:449–454
Dhillon AS, Meikle S, Yazici Z, Eulitz M, Kolch W (2002) Regulation of Raf-1 activation and signalling by dephosphorylation. EMBO J 21:64–71
Zubiaur M, Fernandez O, Ferrero E, Salmeron J, Malissen B, Malavasi F, Sancho J (2002) CD38 is associated with lipid rafts and upon receptor stimulation leads to Akt/protein kinase B and Erk activation in the absence of the CD3-zeta immune receptor tyrosine-based activation motifs. J Biol Chem 277:13–22
He HJ, Kole S, Kwon YK, Crow MT, Bernier M (2003) Interaction of filamin A with the insulin receptor alters insulin-dependent activation of the mitogen-activated protein kinase pathway. J Biol Chem 278:27096–27104
Kujime K, Hashimoto S, Gon Y, Shimizu K, Horie T (2000) p38 mitogen-activated protein kinase and c-jun-NH2-terminal kinase regulate RANTES production by influenza virus-infected human bronchial epithelial cells. J Immunol 164:3222–3228
Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM (1998) Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem 273:18623–18632
Han Z, Boyle DL, Chang L, Bennett B, Karin M, Yang L, Manning AM, Firestein GS (2001) c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J Clin Invest 108:73–81
Vandel L, Montreau N, Vial E, Pfarr CM, Binetruy B, Castellazzi M (1996) Stepwise transformation of rat embryo fibroblasts: c-Jun, JunB, or JunD can cooperate with Ras for focus formation, but a c-Jun-containing heterodimer is required for immortalization. Mol Cell Biol 16:1881–1888
Bollag G, Clapp DW, Shih S, Adler F, Zhang YY, Thompson P, Lange BJ, Freedman MH, McCormick F, Jacks T, Shannon K (1996) Loss of NF1 results in activation of the Ras signaling pathway and leads to aberrant growth in haematopoietic cells. Nat Genet 12:144–148
Zhang YY, Vik TA, Ryder JW, Srour EF, Jacks T, Shannon K, Clapp DW (1998) Nf1 regulates hematopoietic progenitor cell growth and ras signaling in response to multiple cytokines. J Exp Med 187:1893–1902
Guha A, Lau N, Huvar I, Gutmann D, Provias J, Pawson T, Boss G (1996) Ras-GTP levels are elevated in human NF1 peripheral nerve tumors. Oncogene 12:507–513
Sherman LS, Atit R, Rosenbaum T, Cox AD, Ratner N (2000) Single cell Ras-GTP analysis reveals altered Ras activity in a subpopulation of neurofibroma Schwann cells but not fibroblasts. J Biol Chem 275:30740–30745
Farassati F, Pan W, Yamoutpour F, Henke S, Piedra M, Frahm S, Al-Tawil S, Mangrum WI, Parada LF, Rabkin SD, Martuza RL, Kurtz A (2008) Ras signaling influences permissiveness of malignant peripheral nerve sheath tumor cells to oncolytic herpes. Am J Pathol 173:1861–1872
Karin M (1995) The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem 270:16483–16486
Dhandapani KM, Khan MM, Wade FM, Wakade C, Mahesh VB, Brann DW (2007) Induction of transforming growth factor-beta1 by basic fibroblast growth factor in rat C6 glioma cells and astrocytes is mediated by MEK/ERK signaling and AP-1 activation. J Neurosci Res 85:1033–1045
Hirota T, Irie K, Okamoto R, Ikeda W, Takai Y (2005) Transcriptional activation of the mouse Necl-5/Tage4/PVR/CD155 gene by fibroblast growth factor or oncogenic Ras through the Raf-MEK-ERK-AP-1 pathway. Oncogene 24:2229–2235
Todisco A, Takeuchi Y, Urumov A, Yamada J, Stepan VM, Yamada T (1997) Molecular mechanisms for the growth factor action of gastrin. Am J Physiol 273:G891–G898
Buchwalter G, Gross C, Wasylyk B (2004) Ets ternary complex transcription factors. Gene 324:1–14
al-Alawi N, Xu G, White R, Clark R, McCormick F, Feramisco JR (1993) Differential regulation of cellular activities by GTPase-activating protein and NF1. Mol Cell Biol 13:2497–2503
Acknowledgments
This study was supported by the Barbara and Fred Erb Endowed Chair in Cancer Genetics to MAT, as well as to the Cancer Center Support Grant of the Karmanos Cancer Institute, Wayne State University (P30CA022453). The authors are grateful for suggestions on statistical analyses provided by Dr. Judith Abrams.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kraniak, J.M., Sun, D., Mattingly, R.R. et al. The role of neurofibromin in N-Ras mediated AP-1 regulation in malignant peripheral nerve sheath tumors. Mol Cell Biochem 344, 267–276 (2010). https://doi.org/10.1007/s11010-010-0551-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-010-0551-1