Abstract
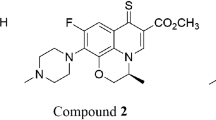
Suberoyl bishydroxamic acid (SBHA) as a histone deacetylase (HDAC) inhibitor has various cellular effects such as cell growth and apoptosis. In the present study, we evaluated the effects of SBHA on the growth and death of A549 lung cancer cells. SBHA inhibited the growth of A549 cells with an IC50 of approximately 50 μM at 72 h in a dose-dependent manner. DNA flow cytometric analysis indicated that SBHA induced a G2/M phase arrest of the cell cycle. This agent also induced apoptosis, as evidenced by sub-G1 cells and annexin V-FITC staining cells. SBHA-induced apoptosis was accompanied by the loss of mitochondrial membrane potential (MMP; ΔΨm), Bcl-2 decrease, Bax increase, and the activation of caspase-3. All of the tested caspase inhibitors significantly rescued some cells from SBHA-induced A549 cell death. However, none of the caspase inhibitors prevented the loss of MMP (ΔΨm) induced by SBHA. Intracellular reactive oxygen species (ROS) levels including O •−2 were increased in 50 μM SBHA-treated A549 cells. None of the caspase inhibitors attenuated ROS levels in these cells. SBHA also elevated the number of glutathione (GSH)-depleted cells in A549 cells, which was reduced by treatment with caspase inhibitors. In conclusion, this is the first report that SBHA inhibited the growth of A549 lung cancer cells via caspase-dependent apoptosis, which was related to GSH depletion rather than changes in ROS level.





Similar content being viewed by others
Abbreviations
- SBHA:
-
Suberoyl bishydroxamic acid
- HDAC:
-
Histone deacetylase
- ROS:
-
Reactive oxygen species
- MMP (ΔΨm):
-
Mitochondrial membrane potential
- FBS:
-
Fetal bovine serum
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PI:
-
Propidium iodide
- FITC:
-
Fluorescein isothiocyanate
- Z-VAD-FMK:
-
Benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone
- Z-DEVD-FMK:
-
Benzyloxycarbonyl-Asp-Glu-Val-Asp-fluoromethylketone
- Z-IETD-FMK:
-
Benzyloxycarbonyl-Ile-Glu-Thr-Asp-fluoromethylketone
- Z-LEHD-FMK:
-
Benzyloxycarbonyl-Leu-Glu-His-Asp-fluoromethylketone
- H2DCFDA:
-
2′,7′-Dichlorodihydrofluorescein diacetate
- DHE:
-
Dihydroethidium
- GSH:
-
Glutathione
- CMFDA:
-
5-Chloromethylfluorescein diacetate
References
Csordas A (1990) On the biological role of histone acetylation. Biochem J 265:23–38
Struhl K (1998) Histone acetylation and transcriptional regulatory mechanisms. Genes Dev 12:599–606
Hassig CA, Schreiber SL (1997) Nuclear histone acetylases and deacetylases and transcriptional regulation: HATs off to HDACs. Curr Opin Chem Biol 1:300–308
Kouzarides T (1999) Histone acetylases and deacetylases in cell proliferation. Curr Opin Genet Dev 9:40–48
Marks PA, Richon VM, Rifkind RA (2000) Histone deacetylase inhibitors: inducers of differentiation or apoptosis of transformed cells. J Natl Cancer Inst 92:1210–1216
Lin RJ, Nagy L, Inoue S, Shao W, Miller WH Jr, Evans RM (1998) Role of the histone deacetylase complex in acute promyelocytic leukaemia. Nature 391:811–814
Al-Janadi A, Chandana SR, Conley BA (2008) Histone deacetylation: an attractive target for cancer therapy? Drugs R D 9:369–383
Lee MJ, Kim YS, Kummar S, Giaccone G, Trepel JB (2008) Histone deacetylase inhibitors in cancer therapy. Curr Opin Oncol 20:639–649
Marks PA, Jiang X (2005) Histone deacetylase inhibitors in programmed cell death and cancer therapy. Cell Cycle 4:549–551
Marchion D, Munster P (2007) Development of histone deacetylase inhibitors for cancer treatment. Expert Rev Anticancer Ther 7:583–598
Cang S, Ma Y, Liu D (2009) New clinical developments in histone deacetylase inhibitors for epigenetic therapy of cancer. J Hematol Oncol 2:22
Marks PA, Xu WS (2009) Histone deacetylase inhibitors: potential in cancer therapy. J Cell Biochem 107:600–608
Frew AJ, Johnstone RW, Bolden JE (2009) Enhancing the apoptotic and therapeutic effects of HDAC inhibitors. Cancer Lett 280:125–133
Eot-Houllier G, Fulcrand G, Magnaghi-Jaulin L, Jaulin C (2009) Histone deacetylase inhibitors and genomic instability. Cancer Lett 274:169–176
Martirosyan A, Leonard S, Shi X, Griffith B, Gannett P, Strobl J (2006) Actions of a histone deacetylase inhibitor NSC3852 (5-nitroso-8-quinolinol) link reactive oxygen species to cell differentiation and apoptosis in MCF-7 human mammary tumor cells. J Pharmacol Exp Ther 317:546–552
Wallach-Dayan SB, Izbicki G, Cohen PY, Gerstl-Golan R, Fine A, Breuer R (2006) Bleomycin initiates apoptosis of lung epithelial cells by ROS but not by Fas/FasL pathway. Am J Physiol Lung Cell Mol Physiol 290:L790–L796
Simon HU, Haj-Yehia A, Levi-Schaffer F (2000) Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 5:415–418
Petty RD, Nicolson MC, Kerr KM, Collie-Duguid E, Murray GI (2004) Gene expression profiling in non-small cell lung cancer: from molecular mechanisms to clinical application. Clin Cancer Res 10:3237–3248
Flis S, Gnyszka A, Splawinski J (2009) HDAC inhibitors, MS275 and SBHA, enhances cytotoxicity induced by oxaliplatin in the colorectal cancer cell lines. Biochem Biophys Res Commun 387:336–341
Ning L, Greenblatt DY, Kunnimalaiyaan M, Chen H (2008) Suberoyl bis-hydroxamic acid activates Notch-1 signaling and induces apoptosis in medullary thyroid carcinoma cells. Oncologist 13:98–104
Zhuang ZG, Fei F, Chen Y, Jin W (2008) Suberoyl bis-hydroxamic acid induces p53-dependent apoptosis of MCF-7 breast cancer cells. Acta Pharmacol Sin 29:1459–1466
Park WH, Seol JG, Kim ES, Hyun JM, Jung CW, Lee CC, Kim BK, Lee YY (2000) Arsenic trioxide-mediated growth inhibition in MC/CAR myeloma cells via cell cycle arrest in association with induction of cyclin-dependent kinase inhibitor, p21, and apoptosis. Cancer Res 60:3065–3071
Han YH, Kim SZ, Kim SH, Park WH (2008) Apoptosis in pyrogallol-treated Calu-6 cells is correlated with the changes of intracellular GSH levels rather than ROS levels. Lung Cancer 59:301–314
Han YH, Kim SW, Kim SH, Kim SZ, Park WH (2008) 2,4-dinitrophenol induces G1 phase arrest and apoptosis in human pulmonary adenocarcinoma Calu-6 cells. Toxicol In Vitro 22:659–670
Park WH, Han YH, Kim SH, Kim SZ (2007) Pyrogallol, ROS generator inhibits As4.1 juxtaglomerular cells via cell cycle arrest of G2 phase and apoptosis. Toxicology 235:130–139
Han YH, Kim SZ, Kim SH, Park WH (2007) Arsenic trioxide inhibits growth of As4.1 juxtaglomerular cells via cell cycle arrest and caspase-independent apoptosis. Am J Physiol Renal Physiol 293:F511–F520
Han YH, Kim SH, Kim SZ, Park WH (2008) Caspase inhibitor decreases apoptosis in pyrogallol-treated lung cancer Calu-6 cells via the prevention of GSH depletion. Int J Oncol 33:1099–1105
Giam M, Huang DC, Bouillet P (2008) BH3-only proteins and their roles in programmed cell death. Oncogene 27(Suppl 1):S128–S136
Poot M, Teubert H, Rabinovitch PS, Kavanagh TJ (1995) De novo synthesis of glutathione is required for both entry into and progression through the cell cycle. J Cell Physiol 163:555–560
Schnelldorfer T, Gansauge S, Gansauge F, Schlosser S, Beger HG, Nussler AK (2000) Glutathione depletion causes cell growth inhibition and enhanced apoptosis in pancreatic cancer cells. Cancer 89:1440–1447
Acknowledgments
This paper was supported by research funds of Chonbuk National University in 2010 and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0007059).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
You, B.R., Park, W.H. Suberoyl bishydroxamic acid inhibits the growth of A549 lung cancer cells via caspase-dependent apoptosis. Mol Cell Biochem 344, 203–210 (2010). https://doi.org/10.1007/s11010-010-0543-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-010-0543-1