Abstract
Cytochrome c (cyt c), a component of the respiratory chain, promotes apoptosis when released into the cytosol. Cyt c anchorage within mitochondria depends on cardiolipin (CL). Detachment and release have been related to CL loss and peroxidation. We report that NaN3-dependent complex IV inhibition, accompanied by impairment of respiration, resulted in cyt c release. Contrarily, inhibition of respiration upstream cyt c with complex I and III inhibitors was not accompanied by the release of the protein, despite CL decrease and monolyso-CL increase. No CL changes and H2O2 formation were observed by inhibiting complex IV. In cyt c–CL liposomes, breaching cyt c–CL hydrophilic interactions produced a higher release of the reduced, compared to the oxidized form, suggesting that the hydrophobic component of cyt c–CL binding is prevalent in the oxidized form. Free or liposome-reconstituted cyt c was able to form fatty acid–protein complexes (palmitate < linoleate < oleate) only in its reduced form. We hypothesize that reduced cyt c–fatty acid binding favors the dislocation of the protein from anchoring CL. A mechanism for cyt c release independent of CL peroxidation by H2O2 is feasible. It could weaken the hydrophobic component of cyt c–CL interactions and might function following complex IV inhibition or in oxygen lack, both conditions producing accumulation of reduced cyt c and free fatty acids.




Similar content being viewed by others
Abbreviations
- Cyt c:
-
Cytochrome c
- S/H buffer:
-
10 mM HEPES (pH 7.4)
- 4 mM KCl:
-
0.1 mM EDTA and 0.30 M sucrose
- CCCP:
-
Carbonyl cyanide 3-chlorophenylhydrazone
- Δψm :
-
Mitochondrial membrane potential
- CL:
-
Cardiolipin
- PC:
-
Phosphatidylcholine
- PE:
-
Phosphatidylethanolamine
- PS:
-
Phosphatidylserine
- PG:
-
Phosphatidylglycerol
References
Wang X (2001) The expanding role of mitochondria in apoptosis. Genes Dev 15:2922–2933
Gogvadze V, Zhivotovsky B (2007) Alteration of mitochondrial function and cell sensitization to death. J Bioenerg Biomembr 39:23–30
Vanderkooi J, Erecinska M, Chance B (1973) Cytochrome c interaction with membranes. I. Use of a fluorescent chromophore in the study of cytochrome c interaction with artificial and mitochondrial membranes. Arch Biochem Biophys 154:219–229
Rytömaa M, Kinnunen PK (1995) Reversibility of the binding of cytochrome c to liposomes. Implications for lipid–protein interactions. J Biol Chem 270:3197–3202
Tuominen EKJ, Wallace CJA, Kinnunen PK (2002) Phospholipid–cytochrome c interaction. Evidence for the extended lipid anchorage. J Biol Chem 277:8822–8826
Kalanxhi E, Wallace CJA (2007) Cytochrome c impaled: investigation of the extended lipid anchorage of a soluble protein to mitochondrial membrane models. Biochem J 407:179–187
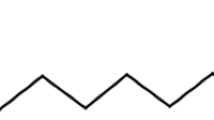
Stewart JM, Blakely JA, Johnson MD (2000) The interaction of ferrocytochrome c with long-chain fatty acids and their CoA and carnitine esters. Biochem Cell Biol 78:675–681
Nantes IL, Zucchi MR, Nascimento OR, Faljoni-Alario A (2001) Effect of heme iron valence state on the conformation of cytochrome c and its association with membrane interfaces. A CD and EPR investigation. J Biol Chem 276:153–158
Monni M, Corazzi L, Migliorati G, Roberti R (2000) Respiratory rate and phosphatidylserine import in brain mitochondria in vitro. J Membr Biol 173:97–105
Piccotti L, Marchetti C, Migliorati G, Roberti R, Corazzi L (2002) Exogenous phospholipids specifically affect transmembrane potential of brain mitochondria and cytochrome c release. J Biol Chem 277:12075–12081
Piccotti L, Buratta M, Giannini S, Gresele P, Roberti R, Corazzi L (2004) Binding and release of cytochrome c in brain mitochondria is influenced by membrane potential and hydrophobic interactions with cardiolipin. J Membr Biol 198:43–53
Macala LJ, Yu RK, Ando S (1983) Analysis of brain lipids by high performance thin-layer chromatography and densitometry. J Lipid Res 24:1243–1250
Petrosillo G, Ruggiero FM, Paradies G (2003) Role of reactive oxygen species and cardiolipin in the release of cytochrome c from mitochondria. FASEB J 17:2202–2208
Liu Y, Fiskum G, Schubert D (2002) Generation of reactive oxygen species by the mitochondrial electron transport chain. J Neurochem 80:780–787
Tretter L, Takas K, Hegedus V, Adam-Vizi V (2007) Characteristics of α-glycerophosphate-evoked H2O2 generation in brain mitochondria. J Neurochem 100:50–663
Schejter A, Luntz TL, Koshy TI, Margoliash E (1992) Relationship between local and global stabilities of proteins: site-direct mutants and chemically-modified derivatives of cytochrome c. Biochemistry 35:8336–8343
Davey GP, Peuchen S, Clark JB (1998) Energy threshold in brain mitochondria. Potential involvement in neurodegeneration. J Biol Chem 273:12753–12757
Vladimirov YA, Proskurnina EV, Izmailov DY, Novikov AA, Brusnichkin AV, Osipov AN, Kagan VE (2006) Cardiolipin activates cytochrome c peroxidase activity since it facilitates H2O2 access to heme. Biochemistry (Mosc) 71:998–1005
Sun D, Gilboe DD (1994) Ischemia-induced changes in cerebral mitochondrial free fatty acids, phospholipids, and respiration in the rat. J Neurochem 62:1921–1928
Macchioni L, Corazzi L, Nardicchi V, Mannucci R, Arcuri C, Porcellati S, Sposini T, Donato R, Goracci G (2004) Rat brain cortex mitochondria release group II secretory phospholipase A2 under reduced membrane potential. J Biol Chem 279:37860–37869
Malhotra A, Edelman-Novemsky I, Xu Y, Plesken H, Ma J, Schlame M, Ren M (2009) Role of calcium-independent phospholipase A2 in the pathogenesis of Barth syndrome. Proc Natl Acad Sci USA 106:2337–2341
Buratta M, Castigli E, Sciaccaluga M, Pellegrino RM, Spinozzi F, Roberti R, Corazzi L (2008) Loss of cardiolipin in palmitate-treated GL15 glioblastoma cells favours cytochrome c release from mitochondria leading to apoptosis. J Neurochem 105:1019–1031
Choi S-Y, Gonzalvez F, Jenkins GM, Slomianny C, Chretien D, Arnoult D, Petit PX, Frohman MA (2007) Cardiolipin deficiency release cytochrome c from the inner mitochondrial membrane and accelerates stimuli-elicited apoptosis. Cell Death Differ 14:597–606
Mileykovskaya E, Dowhan W (2009) Cardiolipin membrane domains in prokaryotes and eukaryotes. Biochim Biophys Acta 1788:2084–2091
Buratta M, Piccotti L, Giannini S, Gresele P, Roberti R, Corazzi L (2006) Selective cytochrome c displacement by phosphate and Ca2+ in brain mitochondria. J Membr Biol 212:199–210
Crompton M (1999) The mitochondrial permeability transition pore and its role in cell death. Biochem J 341:233–249
Nakahara I, Kikuchi H, Taki W, Nishi S, Kito M, Yonekawa Y, Goto Y, Ogata N (1991) Degradation of mitochondrial phospholipids during experimental cerebral ischemia in rats. J Neurochem 57:839–844
Solmaz SRN, Hunte C (2003) Structure of complex III with bound cytochrome c in reduced state and definition of a minimal core interface for electron transfer. J Biol Chem 283:17542–17549
Acknowledgments
This work was supported by grant from Fondazione Cassa di Risparmio di Perugia and from Fondazione Cassa di Risparmio di Terni, Italy. We thank Mr. Carlo Ricci for skillful technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Macchioni, L., Corazzi, T., Davidescu, M. et al. Cytochrome c redox state influences the binding and release of cytochrome c in model membranes and in brain mitochondria. Mol Cell Biochem 341, 149–157 (2010). https://doi.org/10.1007/s11010-010-0446-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-010-0446-1