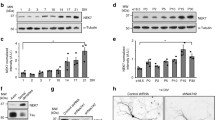
Abstract
FEZ1 was initially described as a neuronal protein that influences axonal development and cell polarization. CLASP2 and NEK1 proteins are present in a centrosomal complex and participate in cell cycle and cell division mechanisms, but their functions were always described individually. Here, we report that NEK1 and CLASP2 colocalize with FEZ1 in a perinuclear region in mammalian cells, and observed that coiled-coil interactions occur between FEZ1/CLASP2 and FEZ1/NEK1 in vitro. These three proteins colocalize and interact with endogenous γ-tubulin. Furthermore, we found that CLASP2 is phosphorylated and interacts with active PKC isoforms, and that FEZ1/CLASP2 colocalization is inhibited by PMA treatment. Our results provide evidence that these three proteins cooperate in centrosomal functions and open new directions for future studies.





Similar content being viewed by others
References
Bloom L, Horvitz HR (1997) The Caenorhabditis elegans gene unc-76 and its human homologs define a new gene family involved in axonal outgrowth and fasciculation. Proc Natl Acad Sci 94:3414–3419
Suzuki T, Okada Y, Semba S, Orba Y, Yamanouchi S, Endo S, Tanaka S, Fujita T, Kuroda S, Nagashima K, Sawa H (2005) Identification of FEZ1 as a protein that interacts with JC virus agnoprotein and microtubules. J Biol Chem 280:24948-24–24948-56
Blasius TL, Cai D, Jih GT, Toret CP, Verhey KJ (2007) Two binding partners cooperate to activate the molecular motor Kinesin-1. J Cell Biol 176:11–17
Ikuta J, Maturana A, Fujita T, Okajima T, Kenji T, Tanizawa K, Kuroda S (2007) Fasciculation and elongation protein zeta-1 (FEZ1) participates in the polarization of hippocampal neuron by controlling the mitochondrial motility. Biochem Biophys Res Commun 353:127–132
Kuroda S, Nakagawa N, Tokunaga C, Tatematsu K, Tanizawa K (1999) Mammalian homologue of the Caenorhabditis elegans UNC-76 protein involved in axonal outgrowth is a protein kinase C zeta-interacting protein. J Cell Biol 114:403–411
Sakae N, Yamasaki N, Kitaichi K, Fukuda T, Yamada M, Yoshikawa H, Hiranita T, Tatsumi Y, Kira JI, Yamamoto T, Miyakawa T, Nakayama KI (2008) Mice lacking the schizophrenia-associated protein FEZ1 manifest hyperactivity and enhanced responsiveness to psychostimulants. Hum Mol Genet 17:3191–3203
Surpili MJ, Delben TM, Kobarg J (2003) Identification of proteins that interact with the central coiled-coil region of the human protein kinase NEK1 involved in the cell cycle regulation. Biochemistry 42:15369–15376
Assmann EM, Alborghetti MR, Camargo ME, Kobarg J (2006) FEZ1 dimerization and interaction with transcription regulatory proteins involves its coiled-coil region. J Biol Chem 281:9869–9881
O’Regan L, Blot J, Fry AM (2007) Mitotic regulation by NIMA-related kinases. Cell Div 29:2–25
Vogler C, Homan S, Pung A, Thorpe C, Barker J, Birkenmeier EH, Upadhya P (1999) Clinical and pathologic findings in two new allelic murine models of polycystic kidney disease. J Am Soc Nephrol 12:2534–2539
Upadhya P, Birkenmeier EH, Birkenmeier CS, Barker JE (2000) Mutations in a NIMA related kinase gene, Nek1, cause pleiotropic effects including a progressive polycystic kidney disease in mice. Proc Natl Acad Sci USA 97:217–221
Mahjoub MR, Trapp ML, Quarmby LM (2005) NIMA-related kinases defective in murine models of polycystic kidney diseases localize to primary cilia and centrosomes. J Am Soc Nephrol 16:3485–3489
Shalom O, Shalva N, Altschuler Y, Motro B (2008) The mammalian Nek1 kinase is involved in primary cilium formation. FEBS Lett 582:1465–1470
White MC, Quarmby LM (2008) The NIMA-family kinase, Nek1 affects the stability of centrosomes and ciliogenesis. BMC Cell Biol 4:9–29
Mimori-Kiyosue Y, Grigoriev I, Lansbergen G, Sasaki H, Matsui C, Severin F, Galjart N, Grosveld F, Vorobjev I, Tsukita S, Akhmanova A (2005) CLASP1 and CLASP2 bind to EB1 and regulate microtubule plus-end dynamics at the cell cortex. J Cell Biol 168:141–153
Kumar P, Lyle KS, Gierke S, Matov A, Danuser G, Wittmann T (2009) GSK3beta phosphorylation modulates CLASP-microtubule association and lamella microtubule attachment. J Cell Biol 184:895–908
Pereira AL, Pereira AJ, Maia AR, Drabek K, Sayas CL, Hergert PJ, Lince Faria M, Matos I, Duque C, Stepanova T, Rieder CL, Earnshaw WC, Galjart N, Maiato H (2006) Mammalian CLASP1 and CLASP2 cooperate to ensure mitotic fidelity by regulating spindle and kinetochore function. Mol Biol Cell 17:4526–4542
Efimov A, Kharitonov A, Efimova N, Loncarek J, Miller PM, Andreyeva N, Gleeson P, Galjart N, Maia AR, McLeod IX, Yates JR, Maiato H, Khodjakov A, Akhmanova A, Kaverina I (2007) Asymmetric CLASP-dependent nucleation of noncentrosomal microtubules at the trans-Golgi network. Dev Cell 12:917–930
Lanza DC, Silva JC, Assmann EM, Quaresma AJ, Bressan GC, Torriani IL, Kobarg J (2009) Human FEZ1 has characteristics of a natively unfolded protein and dimerizes in solution. Proteins 74:104–121
Lanza DC, Trindade DM, Assmann EM, Kobarg J (2008) Over-expression of GFPFEZ1 causes generation of multi-lobulated nuclei mediated by microtubules in HEK293 cells. Exp Cell Res 314:2028–2039
Lupas A, Van Dyke M, Stock J (1991) Predicting coiled-coils from protein sequences. Science 252:1162–1164
Lee S, Walker CL, Karten B, Kuny SL, Tennese AA, O’Neill MA, Wevrick R (2005) Essential role for the Prader–Willi syndrome protein necdin in axonal outgrowth. Hum Mol Genet 14:627–637
Bisgrove BW, Yost HJ (2006) The roles of cilia in developmental disorders and disease. Development 133:4131–4143
Lin F, Hiesberger T, Cordes K, Sinclair AM, Goldstein LSB, Somlo S, Igarashi P (2003) Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Nat Acad Sci 100:5286–5291
Acknowledgements
Financially supported by: Fundação de Amparo à Pesquisa do Estado São Paulo, Conselho Nacional de Pesquisa e Desenvolvimento and LNLS. We thank Maria Eugenia Camargo and Jéssica Santana Bernachi for technical assistance. We are also grateful to Dr. Alexandre J. C. Quaresma for help in providing critical reagents.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11010_2009_317_MOESM1_ESM.tif
Supplementary Fig. 1: The coexpression of GST-NEK1(441–621) or GST-NEK1(497–555) with 6xHis-FEZ1(229–269) results in the elution of a stably interacting complex. 6xHis FEZ1(229–269) and GST only (control), GST-NEK1(441–621) or GST-NEK1(497–555) were produced simultaneously after cotransformation of E. coli BL21 (DE3). The copurifications were performed using a GST-Trap column in an ÄKTATM FPLCTM (GE Healthcare). After mechanical lysis with a French Press, soluble and insoluble fractions were separated by centrifugation and the cleared supernatant was then loaded onto a GST-Trap column pre-equilibrated with lysis buffer (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4, and 1 mM phenylmethylsulfonyl fluoride), followed by extensive wash of the column with the same buffer. Bound proteins were eluted in a gradient of 0–100% of elution buffer (50 mM Tris–HCl and 20 mM reduced gluthatione, pH 8.0). GST only does not copurify with coexpressed 6xHis-FEZ1(229–269). The coexpressions and copurifications are shown by Coomassie stained SDS–PAGE. White arrow heads indicate GST or GST-NEK1 fusion proteins as indicated on top of the figure. Black arrow heads indicate the copurified 6xHis-FEZ1(229–269). St: molecular weight standard proteins. (TIF 1253 kb)
11010_2009_317_MOESM2_ESM.tif
Supplementary Fig. 2: Helical wheel representations of the FEZ1 interaction with NEK1 and CLASP2 coiled-coils. (A, B) Helical wheel representations of predicted hetero-dimeric coiled-coils between FEZ1 (amino acids 229–269) and NEK1 (amino acids 500–555) or CLASP2 (amino acids 1269–1303) heptad repeats. The view is from the N-terminus down to the C-terminus. (TIF 1467 kb)
Rights and permissions
About this article
Cite this article
Lanza, D.C.F., Meirelles, G.V., Alborghetti, M.R. et al. FEZ1 interacts with CLASP2 and NEK1 through coiled-coil regions and their cellular colocalization suggests centrosomal functions and regulation by PKC. Mol Cell Biochem 338, 35–45 (2010). https://doi.org/10.1007/s11010-009-0317-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-009-0317-9