Abstract
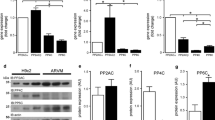
Phospholipase C-β (PLCβ) isozymes (PLCβ1 and PLCβ3) have been extensively characterized in cardiac tissue, but no data are available for the PLCβ4 isozyme. In this study, PLCβ(1–4) isozymes mRNA relative expression was studied by real-time PCR (RT-PCR) in human, rat, and murine left ventricle and the presence of PLCβ4 isozyme at the protein level was confirmed by Western blotting in all species studied. Confocal microscopy experiments carried out in HL-1 cardiomyocytes revealed a sarcoplasmic subcellular distribution of PLCβ4. Although there were unexpected significant interspecies differences in the PLCβ(1–4) mRNA expression, PLCβ4 mRNA was the main transcript expressed in all left ventricles studied. Thus, whereas in human and rat left ventricles PLCβ4 > PLCβ3 > PLCβ2 > PLCβ1 mRNA pattern of expression was found, in murine left ventricle the pattern of expression was different, i.e., PLCβ4 > PLCβ1 > PLCβ3 > PLCβ2. However, results obtained in mouse HL-1 cardiomyocytes showed PLCβ3 ≈ PLCβ4 > PLCβ1 > PLCβ2 pattern of mRNA expression indicating a probable cell type specific expression of the different PLCβ isozymes in cardiomyocytes. Finally, RT-PCR experiments showed a trend, even though not significant (P = 0.067), to increase PLCβ4 mRNA levels in HL-1 cardiomyocytes after angiotensin II treatment. These results demonstrate the presence of PLCβ4 in the heart and in HL-1 cardiomyocytes showing a different species-dependent pattern of expression of the PLCβ(1–4) transcripts. We discuss the relevance of these findings in relation to the development of cardiac hypertrophy.




Similar content being viewed by others
References
Hill JA, Olson EN (2008) Cardiac plasticity. N Engl J Med 358:1370–1380
Lorell BH, Carabello BA (2000) Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation 102:470–479
Clerk A, Sugden PH (1999) Activation of protein kinase cascades in the heart by hypertrophic G protein-coupled receptor agonists. Am J Cardiol 83:64H–69H
Golebiewska U, Scarlata S (2008) Gαq binds two effectors separately in cells: evidence for predetermined signalling pathways. Biophys J 95:2575–2582. doi:10.1529/biophysj.108.129353
Taylor SJ, Chae HZ, Rhee SG, Exton JH (1991) Activation of β1 isozyme of phospholipase C by α subunits of the Gq class of G proteins. Nature 350:516–518. doi:10.1038/350516a0
Filtz TM, Grubb DR, McLeod-Dryden TJ, Luo J, Woodcock EA (2009) Gq-inititated cardiomyocyte hypertrophy is mediated by phospholipase Cβ1b. FASEB J. doi:10.1096/fj.09-133983
Sabri A, Steinberg SF (2003) Protein kinase C isoform-selective signals that lead to cardiac hypertrophy and the progression of heart failure. Mol Cell Biochem 251:97–101. doi:10.1023/A:1025490017780
Luo DL, Gao J, Lan XM, Wang G, Wei S, Xiao RP, Han QD (2006) Role of inositol 1,4,5-trisphosphate receptors in alpha1-adrenergic receptor-induced cardiomyocyte hypertrophy. Acta Pharmacol Sin 27:895–900
Tappia PS (2007) Phospholipid-mediated signaling systems as novel targets for treatment of heart disease. Can J Physiol Pharmacol 85:25–41. doi:10.1139/Y06-098
González-Yanes C, Santos-Alvarez J, Sánchez-Margalet V (2001) Pancreastatin, a chromogranin A-derived peptide, activates Gα16 and phospholipase C-β2 by interacting with specific receptors in rat heart membranes. Cell Signal 13:43–49. doi:10.1016/S0898-6568(00)00127-3
Suh P-G, Park JI, Manzoli L, Cocco L, Peak JC, Katan M, Fukami K, Kataoka T, Yun S, Ryu SH (2008) Multiple roles of phosphoinositide-specific phospholipase C isozymes. BMB Rep 41:415–434
Rebecchi MJ, Pentyala SN (2000) Structure, function and control of phosphoinositide-specific phospholipase C. Physiol Rev 80:1291–1335
Strassheim D, Williams CL (2000) P2Y2 purinergic and M3 muscarinic acetylcholine receptors activate different phospholipase C-beta isoforms that are uniquely susceptible to protein kinase C-dependent phosphorylation and inactivation. J Biol Chem 275:39767–39772. doi:10.1074/jbc.M007775200
Wallace MA, Claro E (1993) Transmembrane signaling through phospholipase C in human cortical membranes. Neurochem Res 18:139–145
Garro MA, López de Jesús M, Ruíz de Azúa I, Callado LF, Meana JJ, Sallés J (2001) Regulation of phospholipase Cβ activity by muscarinic acetylcholine and 5-HT2 receptors in crude and synaptosomal membranes from human cerebral cortex. Neuropharmacology 40:686–695. doi:10.1016/S0028-3908(00)00206-9
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Kawaguchi H, Sano H, Iizuka K, Okada H, Kudo T, Kageyama K, Muramoto S, Murakami T, Okamoto H, Mochizuki N, Kitabatake A (1993) Phosphatidylinositol metabolism in hypertrophic rat heart. Circ Res 72:966–972
Dent M, Dhalla NS, Tappia PS (2004) Phospholipase C gene expression, protein content, and activities in cardiac hypertrophy and heart failure due to volume overload. Am J Physiol Heart Circ Physiol 287:719–727. doi:10.1152/ajpheart.01107.2003
Dent MR, Aroutiounova N, Dhalla NS, Tappia PS (2006) Losartan attenuates phospholipase C isozyme gene expression in hypertrophied hearts due to volume overload. J Cell Mol Med 10:470–479. doi:10.1111/j.1582-4934.2006.tb00412.x
John DY, Lee HH, Park D, Lee CW, Lee KH, Yoo OJ, Rhee SG (1993) Cloning, sequencing, purification and Gq-dependent activation of phospholipase C-β3. J Biol Chem 268:6654–6661
Lee CW, Lee KH, Lee SB, Park D, Rhee SG (1994) Regulation of phospholipase C-β4 by ribonucleotides and the α subunit of Gq. J Biol Chem 269:25335–25338
Lee CW, Park DJ, Lee KH, Kim CG, Rhee SG (1993) Purification, molecular cloning, and sequencing of phospholipase C-β4. J Biol Chem 268:21318–21327
Jiang H, Wu D, Simon MI (1994) Activation of phospholipase C β4 by heterotrimeric GTP-binding proteins. J Biol Chem 269:7593–7596
Rhee SG (2001) Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem 70:281–312. doi:10.1146/annurev.biochem.70.1.281
Kim D, Jun KS, Lee SB, Kang NG, Min DS, Kim YH, Ryu SH, Suh PG, Shin HS (1997) Phospholipase C isozymes selectively couple to specific receptors. Nature 389:290–293. doi:10.1038/38508
Kim MJ, Min DS, Ryu SH, Suh P-G (1998) A cytosolic, Gαq- and βγ-insensitive splice variant of phospholipase C-β4. J Biol Chem 273:3618–3624
Esler M, Kaye D (2000) Measurement of sympathetic nervous system activity in heart failure: the role of norepinephrine kinetics. Heart Fail Rev 5:17–25. doi:10.1023/A:1009889922985
Damy T, Ratajczak P, Shah AM, Camors E, Marty I, Hasenfuss G, Marotte F, Samuel JL, Heymes C (2004) Increased neuronal nitric oxide synthase-derived NO production in the failing human heart. Lancet 363:1365–1367. doi:10.1016/S0140-6736(04)16048-0
Bendall JK, Damy T, Ratajczak P, Loyer X, Monceau V, Marty I, Milliez P, Robidel E, Marotte F, Samuel JL, Heymes C (2004) Role of myocardial neuronal nitric oxide synthase-derived nitric oxide in beta-adrenergic hyporesponsiveness after myocardial infarction-induced heart failure in rat. Circulation 110:2368–2375. doi:10.1161/01.CIR.0000145160.04084.AC
Hilal-Dandan R, Kanter JR, Brunton LL (2000) Characterization of G-protein signaling in ventricular myocytes from the adult mouse heart: differences from the rat. J Mol Cell Cardiol 32:1211–1221. doi:10.1006/jmcc.2000.1156
Sabri A, Pak E, Alcott SA, Wilson B, Steinberg SF (2000) Coupling of endogenous α1- and β-adrenergic receptors in mouse cardiomyocytes. Circ Res 86:1047–1053
Acknowledgments
The authors would like to thank Dr Eneko Urizar for his valuable advice at the time of this manuscript preparation. We would like to thank the cardiac surgeons from Policlinica Guipuzcoa for providing the human left ventricle sample biopsy. We thank to Dr. A. Aiastui for help in immunofluorescence technique; A. Pavón and A. Dorronsoro from Inbiomed for assistance with confocal microscopy This work was supported by the Universidad del País Vasco/Euskal Herriko Unibertsitatea (1/UPV 00079.252-E-15424/2003 to M.A.G.); and the Gobierno Vasco (GV2005111012 to M.A.G.). A.L. holds a fellowship from the Diputacion Foral from Gipuzkoa.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Otaegui, D., Querejeta, R., Arrieta, A. et al. Phospholipase Cβ4 isozyme is expressed in human, rat, and murine heart left ventricles and in HL-1 cardiomyocytes. Mol Cell Biochem 337, 167–173 (2010). https://doi.org/10.1007/s11010-009-0296-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-009-0296-x