Abstract
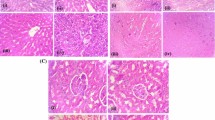
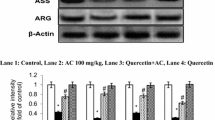
Plant flavonoids are emerging as potent therapeutic drugs effective against a wide range of free radical-mediated diseases. Morin (3,5,7,2′,4′-pentahydroxyflavone), a member of flavonols, is an important bioactive compound by interacting with nucleic acids, enzymes and protein. In this study, we found that morin (30 mg/kg body weight) by oral administration offers protection against hyperammonemia by means of reducing blood ammonia, oxidative stress and enhancing antioxidant status in ammonium chloride-induced (100 mg/kg body weight; i.p) hyperammonemic rats. Enhanced blood ammonia, plasma urea, lipid peroxidation in circulation and tissues (liver and brain) of ammonium chloride-treated rats was accompanied by a significant decrease in the tissues levels of superoxide dismutase (SOD), catalase, reduced glutathione (GSH) and glutathione peroxidase (GPx). Morin administered rats showed a significant reduction in ammonia, urea, lipid peroxidation with a simultaneous elevation in antioxidant levels. Cotreatment with morin prevented the elevation of liver marker enzymes induced by ammonium chloride. The body weight of the animals decreased significantly on ammonium chloride administration when compared with control group. However, cotreatment with morin significantly prevented the decrease of the body weight caused by ammonium chloride. Hyperammonemic rats show liver fibrosis, steatosis, sinusoidal dilatation, etc., along with necrosis, microcystic degeneration in brain. All these changes were reduced in hyperammonemic rats treated with Morin, which too correlated with the biochemical observations. In conclusion, these findings indicate that morin exert antioxidant potential and offer protection against ammonium chloride-induced hyperammonemia. But the exact underlying mechanism needs to be elucidated.


Similar content being viewed by others
References
Monfort P, Felipo V (2005) Long-term potentiation in hippocampus involves sequential activation of soluble guanylate cyclase, cGMP-dependent protein kinase and cGMP-degrading phosphodiesterase, alterations in hyperammonemia. BMC Pharmacol 5:66. doi:10.1186/1471-2210-5-S1-P66
Yu BP (1994) Cellular defenses against damage from reactive oxygen species. Physiol Rev 74:139–162
Halliwell B, Gutteridge JMC (1999) Free radicals in biology and medicine. Oxford University Press, Oxford
Steinberg D, Parathasarathy S, Carew TE, Khow JC, Wiatum JL (1989) Modifications of low density lipoproteins that increase its atherogenicity. N Engl J Med 320:915–918
Hilgier W, Albrecht J, Lisy V, Stastny F (1994) The effect of acute and repeated hyperammonemia on gamma-glutamyl transpeptidase in homogenates and capillaries of various rat brain regions. Mol Chem Neuropathol 13:47–56
Kosenko E, Kaminsky A, Valencia M, Lee L, Hermenegildo C, Felipo V (1997) Superoxide production and antioxidant enzymes in ammonia intoxication in rats. Free Radic Res 27:637–644. doi:10.3109/10715769709097867
Lena PJ, Subramanian P (2004) Effects of melatonin on the levels of antioxidants and lipid peroxidation products in rats treated with ammonium acetate. Pharmazie 59:636–639
Majeed KI (2005) Hyperammonemia is associated with an increase in inhibitory neurotransmission as a consequence of two factors. Med J 2:2–15
Essa MM, Subramanian P (2006) Pongamia pinnata modulates oxidant–antioxidant imbalance during hyperammonemic rats. Fundam Clin Pharmacol 3:299–303. doi:10.1111/j.1472-8206.2006.00410.x
Srinivasan K, Muruganandan S, Lal J, Chandra S, Tandan SK, Prakash VR (2001) Evaluation of anti-inflammatory activity of Pongamia pinnata leaves in rats. J Ethnopharmacol 78:151–157. doi:10.1016/S0378-8741(01)00333-6
Das KD (1994) Naturally occurring flavonoids: structure, chemistry, and high-performance liquid chromatography methods for separation and characterization. Methods Enzymol 234:410–420. doi:10.1016/0076-6879(94)34111-7
Holiman PCH, Hertog MGL, Katanc MB (1996) Analysis and health effects of flavonoids. Food Chem 57:43–46. doi:10.1016/0308-8146(96)00065-9
Harborne JB, Williams CA (2000) Advances in flavonoid research since 1992. Phytochemistry 55:481–504. doi:10.1016/S0031-9422(00)00235-1
Hou YC, Chao PDL, Ho HJ, Wen CC, Hsiu SL (2003) Profound difference in pharmacokinetics between morin and its isomer quercetin in rats. J Pharm Pharmacol 55:199–203. doi:10.1211/002235702487
Windholz M (1989) The Merck index, 11th edn. Merck & Co., Inc., Rahway, pp 986–987
Wijeratne SSK, Abou-Zaid MM, Shahidi F (2006) Antioxidant polyphenols in almond and its coproducts. J Agric Food Chem 54:312–318. doi:10.1021/jf051692j
Aggarwal BB, Shishodia S (2006) Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol 71:1397–1421. doi:10.1016/j.bcp.2006.02.009
Lotito SB, Frei B (2006) Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: cause, consequence, or epiphenomenon? Free Radic Biol Med 41:1727–1746. doi:10.1016/j.freeradbiomed.2006.04.033
Xie MX, Long M, Liu Y, Qin C, Wang YD (2006) Characterisation of the interaction of human serum albumin and morin. Biochim Biophys Acta 1760:1184–1191
Francis AR, Shetty TK, Bhattacharya RK (1989) Modulating effect of plant flavonoids on the mutagenicity of N-methyl-N-nitro-N-nitrosoguanidine. Carcinogenesis 10:1953–1955. doi:10.1093/carcin/10.10.1953
Hanasaki Y, Ogawa S, Fukui S (1994) The correlation between active oxygens scavenging and antioxidative effects of flavonoids. Free Radic Biol Med 16:845–850. doi:10.1016/0891-5849(94)90202-X
Fang SH, Hou YC, Chang WC, Hsiu SL, Chao PD, Chiang BL (2003) Morin sulfates/glucuronides exert anti-inflammatory activity on activated macrophages and decreased the incidence of septic shock. Life Sci 74:743–756. doi:10.1016/j.lfs.2003.07.017
Cook NC, Samman SJ (1996) Flavonoids: chemistry, metabolism, cardioprotective effects and dietary sources. Nutr Biochem 7:66–76. doi:10.1016/0955-2863(95)00168-9
Middleton JRE, Kandaswami C, Theoharides TC (2000) The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease and cancer. Pharmacol Rev 52:673–751
Brown J, O’Prey J, Harrison PR (2003) Enhanced sensitivity of human oral tumours to the flavonol, morin, during cancer progression: involvement of the Akt and stress kinase pathways. Carcinogenesis 24:171–177. doi:10.1093/carcin/24.2.171
Yu ZF, Fong WP, Cheng CHK (2006) The dual actions of morin (3, 5, 7, 2′, 4′-penta-hydroxyl flavone) as a hypouricemic agent: uricosuric effect and xanthine oxidase inhibitory activity. J Pharmacol Exp Ther 316:169–175. doi:10.1124/jpet.105.092684
Cao J, Boucher W, Theoharides TC, Kempuraj D, Madhappan B, Christodoulou S (2005) Flavonoids inhibit proinflammatory mediator release, intracellular calcium ion levels, and protein kinase C theta phosphorylation in human mast cells. Br J Pharmacol 145:934–944. doi:10.1038/sj.bjp.0706268
Kuo HM, Chang LS, Lin YL, Lu HF, Yang JS, Lee JH, Chung (2007) Morin inhibits the growth of human leukemia HL-60 cells via cell cycle arrest and induction of apoptosis through mitochondria dependent pathway. Anticancer Res 27:395–406
Hodek P, Trefil P, Stiborova M (2002) Flavonoids-potent and versatile biologically active compounds interacting with cytochromes P450. Chem Biol Interact 139:1–21. doi:10.1016/S0009-2797(01)00285-X
Lee SF, Lin JK (1997) Inhibitory effects of phytopolyphenols on TPA induced transformation, PKC activation, and c-Jun expression in mouse fibroblast cells. Nutr Cancer 28:177–183
Iwase Y, Takemura Y, Juichi M, Mukainaka T, Ichiishi E, Ito C, Furukawa H, Yano M, Tokuda H, Nishino H (2001) Inhibitory effect of flavonoid derivatives on Epstein-Barr virus activation and two-stage carcinogenesis of skin tumors. Cancer Lett 173:105–109. doi:10.1016/S0304-3835(01)00615-2
Kawabata K, Tanaka T, Honjo S, Kakumoto M, Hara A, Makita H, Tatematsu N, Ushida J, Tsuda H, Mori H (1999) Chemopreventive effect of dietary flavonoid morin on chemically induced rat tongue carcinogenesis. Int J Cancer 83:381–386. doi:10.1002/(SICI)1097-0215(19991029)83:3<381::AID-IJC14>3.0.CO;2-X
Song YM, Kang JW, Zhou J, Wang ZH, Lua XQ, Wang LF, Gao JZ (2000) Study on the fluorescence spectra and electrochemical behavior of ZnL2 and morin with DNA. Spectrochim Acta A Mol Biomol Spectrosc 56:2491–2497. doi:10.1016/S1386-1425(00)00340-1
Cheng CHK (1997) In vitro and in vivo inhibitory actions of morin on rat brain phosphatidylinositolphosphate kinase activity. Life Sci 61:2035–2047. doi:10.1016/S0024-3205(97)00862-X
Subash S, Subramanian P, Sivaperumal R (2007) Antihyperammonemic effect of morin: a dose dependent study. Cell Tissue Res 7:1043–1046
Wolheim DF (1984) Preanalytical increase of ammonia in blood specimens from healthy subjects. Clin Chem 30:906–908
Varley H, Gowenlock AH, Bell M (1998) Practical clinical biochemistry, 4th edn, vol 1. CBS Publishers, New Delhi, pp 161–210
Reitman S, Frankel AS (1957) A colorimetric method for the determination of serum glutatmic oxaloacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28:56–63
King E, Armstrong AR (1934) Determination of serum and bile phosphatase activity. Can Med Assoc J 31:376–378
Yagi K (1987) Lipid peroxides and human disease. Chem Phys Lipids 45:337–351. doi:10.1016/0009-3084(87)90071-5
Fraga CG, Leibovitz BF, Toppel AL (1988) Lipid peroxidation measured as TBARS in tissue slices. Characterization and comparison with homogenate and microsomes. Free Radic Biol Med 4:155–161. doi:10.1016/0891-5849(88)90023-8
Jiang ZY, Hunt JV, Wolff SP (1992) Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxides in low-density lipoprotein. Anal Biochem 202:384–389. doi:10.1016/0003-2697(92)90122-N
Kakkar P, Das B, Viswanathan PN (1984) A modified spectrophotometric assay of SOD. Indian J Biochem Biophys 21:130–132
Sinha KA (1972) Colorimetric assay of catalase. Anal Biochem 47:389–394. doi:10.1016/0003-2697(72)90132-7
Ellman GC (1959) Tissue sulfhydyl groups. Arch Biochem Biophys 82:70–77. doi:10.1016/0003-9861(59)90090-6
Rotruck JT, Pope AL, Ganther HE, Swason AB (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179:588–590. doi:10.1126/science.179.4073.588
Lowry OH, Rosebrough MJ, Farr AL, Randall RJ (1951) Protein measurement with Folin-phenol reagent. J Biol Chem 193:265–275
Nelson DL, Cox MM (2000) Lehninger principles of biochemistry. Macmillan, London
Fedele E, Jin Y, Varnier G, Raiteri M (1996) In vivo microdialysis study of a specific inhibitor of soluble guanylyl cyclase on the glutamate receptor/nitric oxide/cyclic GMP pathway. Br J Pharmacol 119:590–594
Gottlieb M, Leal-Campanario R, Campos-Esparza MR, Sanchez-Gomez MV, Alberdi E, Arranz A, Delqado-Garcia JM, Gruart A, Matutea C (2006) Neuroprotection by two polyphenols following excitotoxicity and experimental ischemia. Neurobiol Dis 23:374–386
Bartosikova L, Necas J, Suchy V, Jankovsa E, Bartosik T, Klusakova J, Jurica J, Florian T, Frydrych M, Krcmar J, Frana P, Franova J (2006) Morin in the therapy of the ischemia-reperfusion damage model of the rat kidney. Ceska Slov Farm 55:78–83
Beal MF (1992) Role of excitotoxicity in human neurological disease. Curr Opin Neurobiol 2:657–662. doi:10.1016/0959-4388(92)90035-J
Kosenko E, Kaminski Y, Lopata O, Muravyov N, Felipo V (1999) Blocking NMDA receptors prevents the oxidative stress induced by acute ammonia intoxication. Free Radic Biol Med 26:1369–1374. doi:10.1016/S0891-5849(98)00339-6
Choi DW (1987) Ionic dependence of glutamate neurotoxicity. J Neurosci 7:369–379
Manev H, Favaron M, Guidotti A, Costa E (1989) Delayed increase of Ca influx elicited by glutamate: role in neuronal death. Mol Pharmacol 36:106–112
Hermenegildo C, Monfort P, Felipo V (2000) Activation of N-methyl-d-aspartate receptors in rat brain in vivo following acute ammonia intoxication: characterization by in vivo brain microdialysis. Hepatology 31:709–715. doi:10.1002/hep.510310322
Hensley K, Tabatabaie T, Stewart CA, Pye Q, Floyd RA (1997) Nitric oxide and derived species as toxic agents in stroke, AIDS dementia, and chronic neurodegenerative disorders. Chem Res Toxicol 10:527–532. doi:10.1021/tx960132z
Schliess F, Gorg B, Fischer R, Desjardins P, Bidmon HJ, Herrmann A, Butterworth RF, Zilles K, Haussinger D (2002) Ammonia induces MK-801-sensitive nitration and phosphorylation of protein tyrosine residues in rat astrocytes. FASEB J 16:739–741
Norenberg MD, Rama Rao KV, Jayakumar AR (2004) Ammonia neurotoxicity and the mitochondrial permeability transition. J Bioenerg Biomembr 36:303–307. doi:10.1023/B:JOBB.0000041758.20071.19
Affany A, Salvayre R, Douste-Blazy L (1987) Comparison of the predictive effect of various flavonoids against lipid peroxidation of erythrocyte membranes (induced by cumen hydroperoxide). Fundam Clin Pharmacol 1:451–457
Kok LDS, Wong YP, Wu TW, Chan HC, Kwok TT, Fung KP (2000) Morin hydrate A potential antioxidant in minimizing the free-radicals-mediated damage to cardiovascular cells by anti-tumor drugs. Life Sci 67:91–99. doi:10.1016/S0024-3205(00)00605-6
Jagetia GC, Rajanikant GK, Rao SK, Baliga MS (2003) Alteration in glutathione, glutathione peroxidase, superoxide dismutase, and lipid peroxidation by ascorbic acid in the skin of mice exposed to fractionated gamma radiation. Clin Chim Acta 332:111–121. doi:10.1016/S0009-8981(03)00132-3
Makris DP, Rossiter JT (2002) Hydroxyl free radical-mediated oxidative degradation of quercetin and morin: a preliminary investigation. J Food Compost Anal 15:103–113. doi:10.1006/jfca.2001.1030
Rahman I, MacNee W (1999) Lung glutathione and oxidative stress: implications in cigarette smoking-induced airway disease. Am J Physiol 277:1067–1088
Jagetia GC, Reddy TK, Kedlaya R (2004) Influence of naringin on ferric iron induced oxidative damage in vitro. Clin Chim Acta 347:189–197. doi:10.1016/j.cccn.2004.04.022
Bartosikova L, Necas J, Suchy V, Kubinova R, Vesela D, Benes L, Bartosik T, Illek J, Salplachta J, Klusakova J, Bartosova L, Strnadova V, Frana P, Franova J (2003) Monitoring of antioxidative effect of morine in alloxan-induced diabetes mellitus in the laboratory rat. Acta Vet Brno 72:191–200
Acknowledgement
S. Subash gratefully acknowledge the financial assistance awarded by the Indian Council of Medical Research (ICMR), New Delhi, India, in the form of Senior Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Subash, S., Subramanian, P. Morin a flavonoid exerts antioxidant potential in chronic hyperammonemic rats: a biochemical and histopathological study. Mol Cell Biochem 327, 153–161 (2009). https://doi.org/10.1007/s11010-009-0053-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-009-0053-1