Abstract
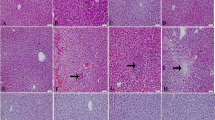
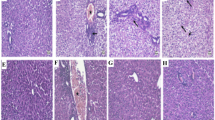
We investigated the regulation of antioxidant system under acetaminophen (AAP) toxicity. Twelve male New Zealand rabbits were divided into two groups with the following treatments: Group 1 animals were intraperitoneally injected with single saline (control). Group 2 animals were treated with intraperitoneal injection of AAP at a dose of 250 mg/kg body weight. Four hours following the treatments, blood samples were collected and the rabbits were sacrificed to collect liver samples. Hepatocellular damage was evaluated by aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels as well as histopathological examinations and immunohistochemical analysis. Tissue-reduced glutathione (GSH), nitric oxide (NO·), and malondialdehyde (MDA) levels were also measured. mRNA expression levels of the antioxidant enzymes superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px) were measured by semi-quantitative RT-PCR. It was found that liver GSH was reduced significantly in AAP-treated rabbits (P < 0.05), while MDA and NO· levels were increased when they were compared to control (P < 0.05). Blood AST and ALT levels were also increased following AAP treatment (P < 0.05). Hepatocellular degeneration and severe necrosis were detected in histopathological examinations. Increased immunostaining was observed for inducible nitric oxide synthase (iNOS) and nitrotyrosine in the liver. There were no changes in mRNA expression levels of SOD, CAT, and GSH-Px after AAP treatment compared to control group. These results suggest that the expression of these enzymes, which are involved in the antioxidant system, may not be altered after AAP toxicity, although classical toxic changes such as depletion of GSH, hepatocellular necrosis, and increased immunostaining for iNOS and nitrotyrosine were detected.





Similar content being viewed by others
References
James LP, Mayeux PR, Hinson JA (2003) Acetaminophen-induced hepatotoxicity. Drug Metab Dispos 31:1499–1506. doi:10.1124/dmd.31.12.1499
Sheen CL, Dillon JF, Bateman DN et al (2002) Paracetamol toxicity: epidemiology, prevention and costs to the health-care system. QJM 95:609–619. doi:10.1093/qjmed/95.9.609
Dahlin DC, Miwa GT, Lu AYH et al (1984) N-Acetyl-para-benzoquinone imine—a cytochrome-P-450-mediated oxidation-product of acetaminophen. Proc Natl Acad Sci USA 81:1327–1331
Nelson SD (1990) Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin Liver Dis 10:267–278
Mitchell JR, Jollow DJ, Potter WZ et al (1973) Acetaminophen-induced hepatic necrosis. 4. Protective role of glutathione. J Pharmacol Exp Ther 187:211–217
Jollow DJ, Mitchell JR, Potter WZ et al (1973) Acetaminophen-induced hepatic necrosis. 2. Role of covalent binding in-vivo. J Pharmacol Exp Ther 187:195–202
Jaeschke H, Bajt ML (2006) Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol Sci 89:31–41. doi:10.1093/toxsci/kfi336
Pumford NR, Roberts DW, Benson RW et al (1990) Immunochemical quantitation of 3-(cystein-S-yl) acetaminophen protein adducts in subcellular liver fractions following a hepatotoxic dose of acetaminophen. Biochem Pharmacol 40:573–579. doi:10.1016/0006-2952(90)90558-3
Knight TR, Kurtz A, Bajt ML et al (2001) Vascular and hepatocellular peroxynitrite formation during acetaminophen toxicity: role of mitochondrial oxidant stress. Toxicol Sci 62:212–220. doi:10.1093/toxsci/62.2.212
Reid AB, Kurten RC, McCullough SS et al (2005) Mechanisms of acetaminophen-induced hepatotoxicity: role of oxidative stress and mitochondrial permeability transition in freshly isolated mouse hepatocytes. J Pharmacol Exp Ther 312:509–516. doi:10.1124/jpet.104.075945
Ito Y, Abril ER, Bethea NW et al (2004) Role of nitric oxide in hepatic microvascular injury elicited by acetaminophen in mice. Am J Physiol Gastrointest Liver Physiol 286:G60–G67. doi:10.1152/ajpgi.00217.2003
Bajt ML, Knight TR, Farhood A et al (2003) Scavenging peroxynitrite with glutathione promotes regeneration and enhances survival during acetaminophen-induced liver injury in mice. J Pharmacol Exp Ther 307:67–73. doi:10.1124/jpet.103.052506
Nakae D, Yamamoto K, Yoshiji H et al (1990) Liposome-encapsulated superoxide dismutase prevents liver necrosis induced by acetaminophen. Am J Pathol 136:787–795
Rodriguez C, Mayo JC, Sainz RM et al (2004) Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res 36:1–9. doi:10.1046/j.1600-079X.2003.00092.x
Cortas NK, Wakid NW (1990) Determination of inorganic nitrate in serum and urine by a kinetic cadmium-reduction method. Clin Chem 36:1440–1443
Ellman G (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77. doi:10.1016/0003-9861(59)90090-6
Mihara M, Uchiyama M (1978) Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 86:271–278. doi:10.1016/0003-2697(78)90342-1
Lowry OH, Rosebrough NJ, Farr AL (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Hiltunen T, Luoma J, Nikkari T et al (1995) Induction of 15-lipoxygenase messenger-RNA and protein in early atherosclerotic lesions. Circulation 92:3297–3303
Jaeschke H (1990) Glutathione disulfide formation and oxidant stress during acetaminophen-induced hepatotoxicity in mice in vivo: the protective effect of allopurinol. J Pharmacol Exp Ther 255:935–941
Rubbo H, Radi R, Trujillo M et al (1994) Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid-peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J Biol Chem 269:26066–26075
O’donnell VB, Fremann BA (2001) Interactions between nitric oxide and lipid oxidation pathways : implications for vascular disease. Circ Res 88:12–21
Grisham MB, Jourd’Heuil D, Wink DA (1999) Nitric oxide. I. Physiological chemistry of nitric oxide and its metabolites: implications in inflammation. Am J Physiol 276:G315–G321
Abdel-Zaher AO, Abdel-Rahman MM, Hafez MM et al (2007) Role of nitric oxide and reduced glutathione in the protective effects of aminoguanidine, gadolinium chloride and oleanolic acid against acetaminophen-induced hepatic and renal damage. Toxicology 234:124–134. doi:10.1016/j.tox.2007.02.014
Hinson JA, Reid AB, McCullough SS et al (2004) Acetaminophen-induced hepatotoxicity: role of metabolic activation, reactive oxygen/nitrogen species, and mitochondrial permeability transition. Drug Metab Rev 36:805–822. doi:10.1081/DMR-200033494
Tirmenstein MA, Nelson SD (1990) Acetaminophen-induced oxidation of protein thiols. Contribution of impaired thiol-metabolizing enzymes and the breakdown of adenine nucleotides. J Biol Chem 265:3059–3065
Lorez Arnaiz S, Llesuy S, Cutrin JC et al (1995) Oxidative stress by acute acetaminophen administration in mouse liver. Free Radic Biol Med 19:303–310. doi:10.1016/0891-5849(95)00023-Q
Mirochnitchenko O, Weisbrot-Lefkowitz M, Reuhl K et al (1999) Acetaminophen toxicity. Opposite effects of two forms of glutathione peroxidase. J Biol Chem 274:10349–10355. doi:10.1074/jbc.274.15.10349
Lei XG, Zhu JH, McClung JP et al (2006) Mice deficient in Cu, Zn-superoxide dismutase are resistant to acetaminophen toxicity. Biochem J 399:455–461. doi:10.1042/BJ20060784
Zhu JH, Zhang X, McClung JP et al (2006) Impact of Cu, Zn-superoxide dismutase and Se-dependent glutathione peroxidase-1 knockouts on acetaminophen-induced cell death and related signaling in murine liver. Exp Biol Med (Maywood) 231:1726–1732
Ruepp SU, Tonge RP, Shaw J et al (2002) Genomics and proteomics analysis of acetaminophen toxicity in mouse liver. Toxicol Sci 65:135–150. doi:10.1093/toxsci/65.1.135
Bhor VM, Raghuram N, Sivakami S (2004) Oxidative damage and altered antioxidant enzyme activities in the small intestine of streptozotocin-induced diabetic rats. Int J Biochem Cell Biol 36:89–97. doi:10.1016/S1357-2725(03)00142-0
Shull S, Heintz NH, Periasamy M et al (1991) Differential regulation of antioxidant enzymes in response to oxidants. J Biol Chem 266:24398–24403
Nishio Y, Kashiwagi A, Taki H et al (1998) Altered activities of transcription factors and their related gene expression in cardiac tissues of diabetic rats. Diabetes 47:1318–1325. doi:10.2337/diabetes.47.8.1318
Hardmeier R, Hoeger H, Fang-Kircher S et al (1997) Transcription and activity of antioxidant enzymes after ionizing irradiation in radiation-resistant and radiation-sensitive mice. Proc Natl Acad Sci USA 94:7572–7576. doi:10.1073/pnas.94.14.7572
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s11010-009-0093-6
An erratum to this article is available at http://dx.doi.org/10.1007/s11010-014-2010-x.
Rights and permissions
About this article
Cite this article
Cigremis, Y., Turel, H., Adiguzel, K. et al. The effects of acute acetaminophen toxicity on hepatic mRNA expression of SOD, CAT, GSH-Px, and levels of peroxynitrite, nitric oxide, reduced glutathione, and malondialdehyde in rabbit. Mol Cell Biochem 323, 31–38 (2009). https://doi.org/10.1007/s11010-008-9961-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-008-9961-8