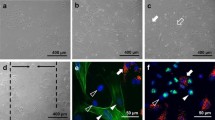
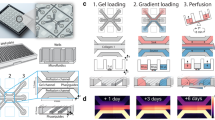
Abstract
Vascular reorganization in wound healing is a complex process, which involves coagulation, endothelial cell proliferation and migration, basement membrane regeneration, and fibrinolysis. During this healing process, the hemostatic system and the angiogenic system are intimately interconnected. To elucidate the contribution of plasminogen in the process of wound healing, we have established a perfusion cell culture system. Using this novel cell culture system, we found that addition of plasminogen in the perfusion medium allowed the “scratch-wounded” endothelial cells to recover completely, while mini-plasminogen only affected the migration but not the proliferation of the endothelial cells. In the process of recovery with the addition of plasminogen, significant plasmin activity could only be detected when the growth of the endothelial cells have almost reached confluence. This finding indicates that wound healing is triggered and promoted during the absence of the proteolytic activity of plasmin. In addition, we could not detect any matrix metalloproteinase activity in the perfusion culture medium throughout the whole culture period. However, we did found that the circulating medium collected from the perfusion system at the early phase of the healing process has stimulatory activity on the growth of endothelial cells, but the proliferative activity decreased back to the basal level when the cells reached confluence. Thus, by using the perfusion cell culture system, we found that proliferation of endothelial cells is regulated by plasminogen and the wound healing process is controlled by a temporal interaction between the endothelial cells and plasminogen.







Similar content being viewed by others
References
Li J, Zhang YP, Kirsner RS (2003) Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microsc Res Tech 60:107–114. doi:10.1002/jemt.10249
Folkman J, Shing Y (1992) Angiogenesis. J Biol Chem 267:10931–10934
O’Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M et al (1994) Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 79:315–328. doi:10.1016/0092-8674(94)90200-3
Dong Z, Kumar R, Yang X, Fidler IJ (1997) Macrophage-derived metalloelastase is responsible for the generation of angiostatin in Lewis lung carcinoma. Cell 88:801–810. doi:10.1016/S0092-8674(00)81926-1
Lijnen HR, Ugwu F, Bini A, Collen D (1998) Generation of an angiostatin-like fragment from plasminogen by stromelysin-1 (MMP-3). Biochemistry 37:4699–4702. doi:10.1021/bi9731798
Gately S, Twardowski P, Stack MS, Patrick M, Boggio L, Cundiff DL et al (1996) Human prostate carcinoma cells express enzymatic activity that converts human plasminogen to the angiogenesis inhibitor, angiostatin. Cancer Res 56:4887–4890
Cao Y, Chen A, An SS, Ji RW, Davidson D, Llinás M (1997) Kringle 5 of plasminogen is a novel inhibitor of endothelial cell growth. J Biol Chem 272:22924–22928. doi:10.1074/jbc.272.36.22924
Lijnen HR, Van Hoef B, Ugwu F, Collen D, Roelants I (2000) Specific proteolysis of human plasminogen by a 24 kDa endopeptidase from a novel Chryseobacterium Sp. Biochemistry 39:479–488. doi:10.1021/bi992014r
Gately S, Twardowski P, Stack MS, Cundiff DL, Grella D, Castellino FJ et al (1997) The mechanism of cancer-mediated conversion of plasminogen to the angiogenesis inhibitor angiostatin. Proc Natl Acad Sci USA 94:10868–11087. doi:10.1073/pnas.94.20.10868
Forsgren M, Råden B, Israelsson M, Larsson K, Hedén LO (1987) Molecular cloning and characterization of a full-length cDNA clone for human plasminogen. FEBS Lett 213:254–260. doi:10.1016/0014-5793(87)81501-6
Pepper MS (2001) Role of the matrix metalloproteinase and plasminogen activator-plasmin systems in angiogenesis. Arterioscler Thromb Vasc Biol 21:1104–1117. doi:10.1161/hq0701.093685
Pins GD, Collins-Pavao ME, Van De Water L, Yarmush ML, Morgan JR (2000) Plasmin triggers rapid contraction and degradation of fibroblast-populated collagen lattices. J Invest Dermatol 114:647–653. doi:10.1046/j.1523-1747.2000.00858.x
Roth D, Piekarek M, Paulsson M, Christ H, Bloch W, Krieg T et al (2006) Plasmin modulates vascular endothelial growth factor-A-mediated angiogenesis during wound repair. Am J Pathol 168:670–684. doi:10.2353/ajpath.2006.050372
Whitelock JM, Murdoch AD, Iozzo RV, Underwood PA (1996) The degradation of human endothelial cell-derived perlecan and release of bound basic fibroblast growth factor by stromelysin, collagenase, plasmin, and heparanases. J Biol Chem 271:10079–10086. doi:10.1074/jbc.271.17.10079
Carmeliet P, Moons L, Dewerchin M, Rosenberg S, Herbert JM, Lupu F et al (1998) Receptor-independent role of urokinase-type plasminogen activator in pericellular plasmin and matrix metalloproteinase proteolysis during vascular wound healing in mice. J Cell Biol 140:233–245. doi:10.1083/jcb.140.1.233
Carmeliet P, Moons L, Ploplis V, Plow E, Collen D (1997) Impaired arterial neointima formation in mice with disruption of the plasminogen gene. J Clin Invest 99:200–208. doi:10.1172/JCI119148
Eitzman DT, McCoy RD, Zheng X, Fay WP, Shen T, Ginsburg D (1996) Bleomycin-induced pulmonary fibrosis in transgenic mice that either lack or overexpress the murine plasminogen activator inhibitor-1 gene. J Clin Invest 97:232–237. doi:10.1172/JCI118396
Romer J, Bugge TH, Pyke C, Lund LR, Flick MJ, Degen JL et al (1996) Impaired wound healing in mice with a disrupted plasminogen gene. Nat Med 2:287–292. doi:10.1038/nm0396-287
Kitching AR, Holdsworth SR, Ploplis VA, Plow EF, Collen D, Carmeliet P et al (1997) Plasminogen and plasminogen activators protect against renal injury in crescentic glomerulonephritis. J Exp Med 185:963–968. doi:10.1084/jem.185.5.963
Dano K, Behrendt N, Brünner N, Ellis V, Ploug M, Pyke C (1994) The urokinase receptor. Protein structure and role in plasminogen activation and cancer invasion. Fibrinolysis 8(Suppl. 1):189–203. doi:10.1016/0268-9499(94)90717-X
Singer AJ, Clark RA (1999) Cutaneous wound healing. N Engl J Med 341:738–746. doi:10.1056/NEJM199909023411006
Li WY, Chong SS, Huang EY, Tuan TL (2003) Plasminogen activator/plasmin system: a major player in wound healing? Wound Repair Regen 11:239–247. doi:10.1046/j.1524-475X.2003.11402.x
Hertig A, Rondeau E (2004) Role of the coagulation/fibrinolysis system in fibrin-associated glomerular injury. J Am Soc Nephrol 15:844–853. doi:10.1097/01.ASN.0000115400.52705.83
Minuth WW (1991) MINUSHEET—a new method for “natural” culture conditions of epithelia in vitro. ALTEX 8:18–30
Sanzo MA, Marasa JC, Wittwer AJ, Siegel NR, Harakas NK, Feder J (1987) Purification and characterization of a tissue plasminogen activator-inhibitor complex from human umbilical vein endothelial cell conditioned medium. Biochemistry 26:7443–7449. doi:10.1021/bi00397a037
Hayashi M, Tamura Y, Dohmae N, Kojima S, Shimonaka M (2008) Plasminogen N-terminal activation peptide modulates the activity of angiostatin-related peptides on endothelial cell proliferation and migration. Biochem Biophys Res Commun 369:635–640. doi:10.1016/j.bbrc.2008.02.050
O’Neil CH, Boffa MB, Hancock MA, Pickering JG, Koschinsky ML (2004) Stimulation of vascular smooth muscle cell proliferation and migration by apolipoprotein(a) is dependent on inhibition of transforming growth factor-b activation and on the presence of kringle IV type 9. J Biol Chem 279:55187–55195. doi:10.1074/jbc.M409860200
Herron GS, Banda MJ, Clark EJ, Gavrilovic J, Werb Z (1986) Secretion of metalloproteinases by stimulated capillary endothelial cells. II. Expression of collagenase and stromelysin activities is regulated by endogenous inhibitors. J Biol Chem 261:2814–2818
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. doi:10.1038/227680a0
Levin EG, Loskutoff DJ (1982) Cultured bovine endothelial cells produce both urokinase and tissue-type plasminogen activators. J Cell Biol 94:631–636. doi:10.1083/jcb.94.3.631
van Mourik JA, Lawrence DA, Loskutoff DJ (1984) Purification of an inhibitor of plasminogen activator (antiactivator) synthesized by endothelial cells. J Biol Chem 259:14914–14921
Zucker S, Conner C, DiMassmo BI, Ende H, Drews M, Seiki M et al (1995) Thrombin induces the activation of progelatinase A in vascular endothelial cells. Physiologic regulation of angiogenesis. J Biol Chem 270:23730–23738. doi:10.1074/jbc.270.40.23730
Lafleur MA, Hollenberg MD, Atkinson SJ, Knäuper V, Murphy G, Edwards DR (2001) Activation of pro-(matrix metalloproteinase-2) (pro-MMP-2) by thrombin is membrane-type-MMP-dependent in human umbilical vein endothelial cells and generates a distinct 63 kDa active species. Biochem J 357:107–115. doi:10.1042/0264-6021:3570107
Davis GE, Pintar Allen KA, Salazar R, Maxwell SA (2001) Matrix metalloproteinase-1 and -9 activation by plasmin regulates a novel endothelial cell-mediated mechanism of collagen gel contraction and capillary tube regression in three-dimensional collagen matrices. J Cell Sci 114:917–930
Booyse FM, Scheinbuks J, Lin PH, Traylor M, Bruce R (1988) Isolation and interrelationships of the multiple molecular tissue-type and urokinase-type plasminogen activator forms produced by cultured human umbilical vein endothelial cells. J Biol Chem 263:15129–15138
Levin EG (1983) Latent tissue plasminogen activator produced by human endothelial cells in culture: Evidence for an enzyme-inhibitor complex. Proc Natl Acad Sci USA 80:6804–6808. doi:10.1073/pnas.80.22.6804
Chapman HA (1997) Plasminogen activators, integrins, and the coordinated regulation of cell adhesion and migration. Curr Opin Cell Biol 9:714–724. doi:10.1016/S0955-0674(97)80126-3
Vogten JM, Reijerkerk A, Meijers JC, Voest EE, Borel Rinkes IH, Gebbink MF (2003) The role of the fibrinolytic system in corneal angiogenesis. Angiogenesis 6:311–316. doi:10.1023/B:AGEN.0000029414.24060.fe
Leksa V, Godar S, Schiller HB, Fuertbauer E, Muhammad A, Slezakova K et al (2005) TGF-β-induced apoptosis in endothelial cells mediated by M6P/IGFII-R and mini-plasminogen. J Cell Sci 118:4577–4586. doi:10.1242/jcs.02587
Duboscq C, Genoud V, Parborell MF, Kordich LC (1997) Impaired clot lysis by rt-PA catalyzed mini-plasminogen activation. Thromb Res 86:505–513. doi:10.1016/S0049-3848(97)00099-6
Wu HL, Wu IS, Fang RY, Hau JS, Wu DH, Chang BI et al (1992) The binding of plasminogen fragments to cultured human umbilical vein endothelial cells. Biochem Biophys Res Commun 188:703–711. doi:10.1016/0006-291X(92)91113-5
Goretzki L, Lombardo CR, Stallcup WB (2000) Binding of the NG2 proteoglycan to kringle domains modulates the functional properties of angiostatin and plasmin(ogen). J Biol Chem 275:28625–28633. doi:10.1074/jbc.M002290200
Narasaki R, Kuribayashi H, Shimizu K, Imamura D, TSato T, Hasumi K (2005) Bacillolysin MA, a novel bacterial metalloproteinase that produces angiostatin-like fragments from plasminogen and activates protease zymogens in the coagulation and fibrinolysis systems. J Biol Chem 280:14278–14287. doi:10.1074/jbc.M500241200
Morikawa W, Yamamoto K, Ishikawa S, Takemoto S, Ono M, Fukushi J et al (2000) Angiostatin generation by cathepsin D secreted by human prostate carcinoma cells. J Biol Chem 275:38912–38920. doi:10.1074/jbc.M005402200
Bykowska K, Levin EG, Rijken DC, Loskutoff DJ, Collen D (1982) Characterization of a plasminogen activator secreted by cultured bovine aortic endothelial cells. Biochim Biophys Acta 703:113–115
Rijken DC, van Hinsbergh VW, Sens EH (1984) Quantitation of tissue-type plasminogen activator in human endothelial cell cultures by use of an enzyme immunoassay. Thromb Res 33:145–153. doi:10.1016/0049-3848(84)90175-0
Sprengers ED, Verheijen JH, Van Hinsbergh VW, Emeis JJ (1984) Evidence for the presence of two different fibrinolytic inhibitors in human endothelial cell conditioned medium. Biochim Biophys Acta 801:163–170
Wu HL, Chang BI, Wu DH, Chang LC, Gong CC, Lou KL et al (1990) Interaction of plasminogen and fibrin in plasminogen activation. J Biol Chem 265:19658–19664
Nagase H, Woessner JF Jr (1999) Matrix metalloproteinases. J Biol Chem 274:21491–21494. doi:10.1074/jbc.274.31.21491
Mimuro J, Schleef RR, Loskutoff DJ (1987) Extracellular matrix of cultured bovine aortic endothelial cells contains functionally active type 1 plasminogen activator inhibitor. Blood 70:721–728
Declerck PJ, De Mol M, Alecci MC, Baudner S, Paques EP, Preissner KT et al (1988) Purification and characterization of a plasminogen activator inhibitor 1 binding protein from human plasma. Identification as a multifunctional form of S protein (vitronectin). J Biol Chem 263:15454–15461
Devy L, Blacher S, Grignet-Debrus C, Bajou K, Masson V, Gerard RD et al (2002) The pro- or antiangiogenic effect of plasminogen activator inhibitor 1 is dose dependent. FASEB J 16:147–154. doi:10.1096/fj.01-0552com
Hasenstab D, Lea H, Clowes AW (2000) Local plasminogen activator inhibitor type 1 overexpression in rat carotid artery enhances thrombosis and endothelial regeneration while inhibiting intimal thickening. Arterioscler Thromb Vasc Biol 20:853–859
Acknowledgment
We are deeply grateful to Dr. Nicholas Ling, Neurocrine Biosciences, for critical reading of the manuscript and helpful advice and discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hayashi, M., Matsuzaki, Y. & Shimonaka, M. Impact of plasminogen on an in vitro wound healing model based on a perfusion cell culture system. Mol Cell Biochem 322, 1–13 (2009). https://doi.org/10.1007/s11010-008-9934-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-008-9934-y