Abstract
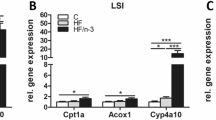
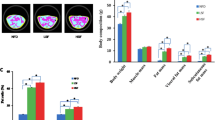
Liver fatty acid binding protein (L-FABP) is highly expressed in both enterocytes and hepatocytes and binds multiple ligands, including saturated (SFA), unsaturated fatty acids (PUFA), and cholesterol. L-fabp −/− mice were protected against obesity and hepatic steatosis on a high saturated fat (SF), high cholesterol “Western” diet and manifested a similar phenotype when fed with a high SF, low cholesterol diet. There were no significant differences in fecal fat content or food consumption between the genotypes, and fatty acid (FA) oxidation was reduced, rather than increased, in SF-fed L-fabp −/− mice as evidenced by decreased heat production and serum ketones. In contrast to mice fed with a SF diet, L-fabp −/− mice fed with a high PUFA diet were not protected against obesity and hepatic steatosis. These observations together suggest that L-fabp −/− mice exhibit a specific defect in the metabolism of SFA, possibly reflecting altered kinetics of FA utilization. In support of this possibility, microarray analysis of muscle from Western diet-fed mice revealed alterations in genes regulating glucose uptake and FA synthesis. In addition, intestinal cholesterol absorption was decreased in L-fabp −/− mice. On the other hand, and in striking contrast to other reports, female L-fabp −/− mice fed with low fat, high cholesterol diets gained slightly less weight than control mice, with minor reductions in hepatic triglyceride content. Together these data indicate a role for L-FABP in intestinal trafficking of both SFA and cholesterol.


Similar content being viewed by others
References
Chmurzynska A (2006) The multigene family of fatty acid-binding proteins (FABPs): function, structure and polymorphism. J Appl Genet 47:39–48
Cistola DP, Sacchettini JC, Banaszak LJ et al (1989) Fatty acid interactions with rat intestinal and liver fatty acid-binding proteins expressed in Escherichia coli. A comparative 13C NMR study. J Biol Chem 264:2700–2710
Cistola DP, Sacchettini JC, Gordon JI (1990) 13C NMR studies of fatty acid-protein interactions: comparison of homologous fatty acid-binding proteins produced in the intestinal epithelium. Mol Cell Biochem 98:101–110. doi:10.1007/BF00231373
Thompson J, Winter N, Terwey D et al (1997) The crystal structure of the liver fatty acid-binding protein. A complex with two bound oleates. J Biol Chem 272:7140–7150. doi:10.1074/jbc.272.11.7140
Smith AJ, Thompson BR, Sanders MA et al (2007) Interaction of the adipocyte fatty acid-binding protein with the hormone-sensitive lipase: regulation by fatty acids and phosphorylation. J Biol Chem 282:32424–32432. doi:10.1074/jbc.M703730200
Gordon JI, Elshourbagy N, Lowe JB et al (1985) Tissue specific expression and developmental regulation of two genes coding for rat fatty acid binding proteins. J Biol Chem 260:1995–1998
Lowe JB, Sacchettini JC, Laposata M et al (1987) Expression of rat intestinal fatty acid-binding protein in Escherichia coli: purification and comparison of ligand binding characteristics with that of Escherichia coli-derived rat liver fatty acid-binding protein. J Biol Chem 262:5931–5937
Nemecz G, Hubbell T, Jefferson JR et al (1991) Interaction of fatty acids with recombinant rat intestinal and liver fatty acid-binding proteins. Arch Biochem Biophys 286:300–309. doi:10.1016/0003-9861(91)90044-J
Thumser AE, Wilton DC (1996) The binding of cholesterol and bile salts to recombinant rat liver fatty acid-binding protein. Biochem J 320(Pt 3):729–733
Haunerland N, Jagschies G, Schulenberg H et al (1984) Fatty-acid-binding proteins: occurrence of two fatty-acid-binding proteins in bovine liver cytosol and their binding of fatty acids, cholesterol, and other lipophilic ligands. Hoppe Seylers Z Physiol Chem 365:365–376
Wolfrum C, Ellinghaus P, Fobker M et al (1999) Phytanic acid is ligand and transcriptional activator of murine liver fatty acid binding protein. J Lipid Res 40:708–714
Martin GG, Huang H, Atshaves BP et al (2003) Ablation of the liver fatty acid binding protein gene decreases fatty acyl CoA binding capacity and alters fatty acyl CoA pool distribution in mouse liver. Biochem 42:11520–11532. doi:10.1021/bi0346749
Woodford JK, Behnke WD, Schroeder F (1995) Liver fatty acid binding protein enhances sterol transfer by membrane interaction. Mol Cell Biochem 152:51–62
Nemecz G, Schroeder F (1991) Selective binding of cholesterol by recombinant fatty acid binding proteins. J Biol Chem 266:17180–17186
Luxon BA, Milliano MT, Weisiger RA (2000) Induction of hepatic cytosolic fatty acid binding protein with clofibrate accelerates both membrane and cytoplasmic transport of palmitate. Biochim Biophys Acta 1487:309–318
Murphy EJ (1998) L-FABP and I-FABP expression increase NBD-stearate uptake and cytoplasmic diffusion in L cells. Am J Physiol 275:G244–G249
Wolfrum C, Buhlmann C, Rolf B et al (1999) Variation of liver-type fatty acid binding protein content in the human hepatoma cell line HepG2 by peroxisome proliferators and antisense RNA affects the rate of fatty acid uptake. Biochim Biophys Acta 1437:194–201
Newberry EP, Xie Y, Kennedy S et al (2003) Decreased hepatic triglyceride accumulation and altered fatty acid uptake in mice with deletion of the liver fatty acid-binding protein gene. J Biol Chem 278:51664–51672. doi:10.1074/jbc.M309377200
Martin GG, Atshaves BP, McIntosh AL et al (2006) Liver fatty acid binding protein gene ablation potentiates hepatic cholesterol accumulation in cholesterol-fed female mice. Am J Physiol Gastrointest Liver Physiol 290:G36–G48. doi:10.1152/ajpgi.00510.2004
Newberry EP, Xie Y, Kennedy SM et al (2006) Protection against Western diet-induced obesity and hepatic steatosis in liver fatty acid-binding protein knockout mice. Hepatology 44:1191–1205. doi:10.1002/hep.21369
Newberry EP, Kennedy SM, Xie Y et al (2007) Diet-induced obesity and hepatic steatosis in L-FABP−/− mice is abrogated with saturated but not polyunsaturated fat feeding and attenuated following cholesterol supplementation. Am J Physiol Gastrointest Liver Physiol 294:G307–G314. doi:10.1152/ajpgi.00377.2007
Martin GG, Danneberg H, Kumar LS et al (2003) Decreased liver fatty acid binding capacity and altered liver lipid distribution in mice lacking the liver fatty acid-binding protein gene. J Biol Chem 278:21429–21438. doi:10.1074/jbc.M300287200
Xie Y, Newberry EP, Young SG et al (2006) Compensatory increase in hepatic lipogenesis in mice with conditional intestine-specific Mttp deficiency. J Biol Chem 281:4075–4086. doi:10.1074/jbc.M510622200
Wang DQ, Paigen B, Carey MC (2001) Genetic factors at the enterocyte level account for variations in intestinal cholesterol absorption efficiency among inbred strains of mice. J Lipid Res 42:1820–1830
Carlier H, Bernard A, Caselli C (1991) Digestion and absorption of polyunsaturated fatty acids. Reprod Nutr Dev 31:475–500. doi:10.1051/rnd:19910501
Penner G, Gang G, Sun X et al (2002) C/EBP DNA-binding activity is upregulated by a glucocorticoid-dependent mechanism in septic muscle. Am J Physiol Regul Integr Comp Physiol 282:R439–R444
Menconi M, Fareed M, O’Neal P et al (2007) Role of glucocorticoids in the molecular regulation of muscle wasting. Crit Care Med 35:S602–S608. doi:10.1097/01.CCM.0000279194.11328.77
Yang H, Mammen J, Wei W et al (2005) Expression and activity of C/EBPbeta and delta are upregulated by dexamethasone in skeletal muscle. J Cell Physiol 204:219–226. doi:10.1002/jcp.20278
Neeli I, Siddiqi SA, Siddiqi S et al (2007) Liver fatty acid-binding protein initiates budding of pre-chylomicron transport vesicles from intestinal endoplasmic reticulum. J Biol Chem 282:17974–17984. doi:10.1074/jbc.M610765200
Schwarz M, Davis DL, Vick BR et al (2001) Genetic analysis of intestinal cholesterol absorption in inbred mice. J Lipid Res 42:1801–1811
Keelan M, Hui DY, Wild G et al (2000) Variability of the intestinal uptake of lipids is genetically determined in mice. Lipids 35:833–837. doi:10.1007/S11745-000-0592-0
Taylor BA, Wnek C, Schroeder D et al (2001) Multiple obesity QTLs identified in an intercross between the NZO (New Zealand obese) and the SM (small) mouse strains. Mamm Genome 12:95–103. doi:10.1007/s003350010254
Anunciado RV, Ohno T, Mori M et al (2000) Distribution of body weight, blood insulin and lipid levels in the SMXA recombinant inbred strains and the QTL analysis. Exp Anim 49:217–224. doi:10.1538/expanim.49.217
Suto J, Matsuura S, Imamura K et al (1998) Genetics of obesity in KK mouse and effects of A(y) allele on quantitative regulation. Mamm Genome 9:506–510. doi:10.1007/s003359900809
Ueda H, Ikegami H, Kawaguchi Y et al (1999) Genetic analysis of late-onset type 2 diabetes in a mouse model of human complex trait. Diabetes 48:1168–1174. doi:10.2337/diabetes.48.5.1168
Acknowledgments
This work was supported by grants from the NIH to NOD (HL-38180, DK-56260, and DK-52574, particularly the Genomics and Microarray Core). The authors acknowledge Trey Coleman and the Clinical Nutrition Research Unit for assistance with the energy consumption studies (NIDDK P30 DK-56341). The authors acknowledge the Diabetes Research Training Center Immunoassay Core for help with serum analysis (P60 DK-020579-30). The authors are grateful to Valerie Blanc, Kim Delaney, Britni Sternard, and Susie Stanley for useful discussions throughout the course of these studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Newberry, E.P., Kennedy, S.M., Xie, Y. et al. Diet-induced alterations in intestinal and extrahepatic lipid metabolism in liver fatty acid binding protein knockout mice. Mol Cell Biochem 326, 79–86 (2009). https://doi.org/10.1007/s11010-008-0002-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-008-0002-4