Abstract
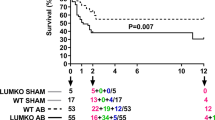
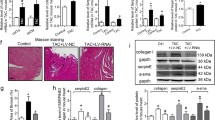
Objective Dilated cardiomyopathy (DCM) represents a large subset of patients with congestive heart failure (HF), and myocardial fibrosis has been shown to be associated with this process. Lysyl oxidase (LOX), a key enzyme, plays a potential role in the biogenesis of connective tissue matrices by catalyzing crosslinks in collagen and elastin. However, the mechanisms involved in the remodeling process during HF are not clearly understood. The present work was aimed to determine the changes in collagen phenotypes, MMPs, TIMPs, and LOX, in DCM and non-failing human hearts. Moreover, the role of TGFβ in the induction of type III collagen in cardiac fibroblast is determined. Method Protein and RNA expression were quantified by Western and RT-PCR analysis; collagen phenotypes were determined by SDS-PAGE. Results Our data demonstrated that in all DCM hearts, the collagen concentration was significantly elevated compared to that of the NF hearts associated with an increase in Type I (18%) and Type III (33%) collagen. The content of MMP-2 and MMP-9 were increased significantly in all DCM hearts compared to NF hearts. Transcriptional level of LOX, TIMP 1, and 2 were significantly upregulated in DCM hearts. In addition, a significant increase in the transcript levels of cytokines, notably IFN, IL-6, TNF-α, and TGF-β superfamily was observed in all DCM hearts. Addition of TGFβ to cardiac fibroblasts caused a dose dependent increase in type III collagen. Conclusion Altogether, our data suggest an alteration of collagen, MMPs, various cytokines and particularly, LOX participates, in part, in the remodeling of the heart leading to cardiac dysfunction and HF.






Similar content being viewed by others
References
Anversa P, Kajstura J, Olivetti G (1996) Myocyte death in heart failure. Curr Opin Cardiol 3:245–251
Colucci WS (1997) Molecular and cellular mechanisms of myocardial failure. Am J Cardiol 4;80(11A):15L–25L
Bishop JE (1998) Regulation of cardiovascular collagen deposition by mechanical forces. Mol Med Today 2:69–75
Hein S, Kostin S, Heling A, Maeno Y, Schaper J (2000) The role of the cytoskeleton in heart failure. Cardiovasc Res 14;45(2):273–278
Mittmann C, Eschenhagen T, Scholz H (1998) Cellular and molecular aspects of contractile dysfunction in heart failure: Cardiovasc Res (2):267–275
Host NB, Stoltenberg MB, Jensen LT, Larsen OG, Aurup P (1995) Effect on collagen metabolism of thrombolytic therapy with tissue-plasminogen activator. A randomized, placebo-controlled study. Eur J Clin Invest 15:18
Marijianowski MM, Teeling P, Mann J, Becker AE (1995) Dilated cardiomyopathy is associated with an increase in the type I/type III collagen ratio: a quantitative assessment. J Am Coll Cardiol 25:1263–1272
Lapiere CM, Nusgens B, Pierard GE (1997) Interaction between collagen type I and type III in conditioning bundles organization. Connect Tissue Res 5(1):21–29
Weber KT, Pick R, Janicki JS, Gadodia G, Lakier JB (1998) Inadequate collagen tethers in dilated cardiopathy. Am Heart J 116(6 Pt 1):1641–1646
Wu Y, Tobias AH, Bell K, Barry W, Helmes M, Trombitas K, Tucker R, Campbell KB, Granzier HL (2004) Cellular and molecular mechanisms of systolic and diastolic dysfunction in an avian model of dilated cardiomyopathy. J Mol Cell Cardiol 37(1):111–119
Norton GR, Tsotetsi J, Trifunovic B, Hartford C, Candy GP, Woodiwiss AJ (1997) Myocardial stiffness is attributed to alterations in cross-linked collagen rather than total collagen or phenotypes in spontaneously hypertensive rats. Circulation 96:1991–1998
Woodiwiss AJ, Tsotetsi OJ, Sprott S, Lancaster EJ, Mela T, Chung ES, Meyer TE, Norton GR (2001) Reduction in myocardial collagen cross-linking parallels left ventricular dilatation in rat models of systolic chamber dysfunction. Circulation 2;103(1):155–160
Li YY, McTiernan CF, Feldman AM (2000) Interplay of matrix metalloproteinases, tissue inhibitors of metalloproteinases and their regulators in cardiac matrix remodeling. Cardiovasc Res 46(2):214–224
Alexander CM, Werb Z (1989) Proteinases and extracellular matrix remodeling. Curr Opin Cell Biol 1(5):974–982
Factor SM, Butany J, Sole MJ, Wigle ED, Williams WC, Rojkind M (1991) Pathologic fibrosis and matrix connective tissue in the subaortic myocardium of patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 17(6):1343–1351
Deten A, Holzl A, Leicht M, Barth W, Zimmer HG (2001) Changes in extracellular matrix and in transforming growth factor beta isoforms after coronary artery ligation in rats. J Mol Cell Cardiol 33(6):1191–1207
Smith-Mungo LI, Kagan HM (1998) Lysyl oxidase: properties, regulation and multiple functions in biology. Matrix Bio l16(7):387–398
Maki JM, Rasanen J, Tikkanen H, Sormunen R, Makikallio K, Kivirikko KI, Soininen R (2002) Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation 5;106(19):2503–2509
Yang CM, Kandaswamy V, Young D, Sen S (1997) Changes in collagen phenotypes during progression and regression of cardiac hypertrophy. Cardiovasc Res 36:236–245
Pathak M, Sarkar S, Vellaichamy E, Sen S (2001) Role of myocytes in myocardial collagen production. Hypertension 37(3):833–840
Bergman J, Loxley R (1963) Two improved and simplified methods for the spectrophotometric determination of hydroxyproline. Anal Chem 35:1961–1965
Mukherjee D, Sen S (1991) Alterations of collagen phenotypes in ischemic cardiomyopathy. J Clin Invest 88:1141–1146
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159
Spinale FG, Coker ML, Thomas CV, Walker JD, Mukherjee R, Hebbar L (1998) Time-dependent changes in matrix metalloproteinase activity and expression during the progression of congestive heart failure: relation to ventricular and myocyte function. Circ Res 9;82(4):482–495
Sil P, Sen S (1997) Angiotensin II and myocyte growth: role of fibroblasts. Hypertension 30(2 Pt 1):209–216
Hornstra IK, Birge S, Starcher B, Bailey AJ, Mecham RP, Shapiro SD (2003) Lysyl oxidase is required for vascular and diaphragmatic development in mice. J Biol Chem 18;278(16):14387–14393
McCormick RJ, Musch TI, Bergman BC, Thomas DP (1994) Regional differences in LV collagen accumulation and mature cross-linking after myocardial infarction in rats. Am J Physiol 266(1 Pt 2):H354–H359
Kato S, Spinale FG (1995) Inhibition of collagen crosslinking: effects on fibrilar collagen and ventricular diastolic function. Am J Physiol 269:H863–H868
Border WA, Noble NA (1994) Transforming growth factor beta in tissue fibrosis. N Engl J Med 10;331(19):1286–1292
Villareal FJ, Dillman WH (1992) Cardiac hypertrohy-induced changes in mRNA levels for TGF-b1, fibronectin and collagen. Am J Physiol 262:H1861–H1866
Hao J, Ju H, Zhao S, Junaid A, Scammell-La Fleur T, Dixon IM (1999) Elevation of expression of Smads 2, 3, and 4, decorin and TGF-beta in the chronic phase of myocardial infarct scar healing. J Mol Cell Cardiol 31(3):667–678
Hao J, Wang B, Jones SC, Jassal DS, Dixon MC (2001) Interaction between angiotensin II and Smad proteins in fibroblasts in failing heart and in vitro. Am J Physiol 279:H3020–H3030
Ritzenthaler JD, Goldstein RH, Fine A, Lichtler A, Rowe DW, Smith BD (1991) Transforming-growth-factor-beta activation elements in the distal promoter regions of the rat alpha 1 type I collagen gene. Biochem J 15;280(Pt 1):157–162
Rossi P, Karsenty G, Roberts AB, Roche NS, Sporn MB, De CB (1988)A nuclear factor 1 binding site mediates the transcriptional activation of a type I collagen promoter by transforming growth factor-beta. Cell 12;52(3):405–414
Sarkar S, Vellaichamy E, Young D, Sen S (2004) Influence of cytokines and growth factors in ANG II-mediated collagen upregulation by fibroblasts in rats: role of myocytes. Am J Physiol Heart Circ Physiol 287(1):H107–H117
Butt RP, Laurent GJ, Bishop JE (1995) Collagen production and replication by cardiac fibroblasts is enhanced in response to diverse classes of growth factors. Eur J Cell Biol 68:330–335
Bashey RI, Donnelly M, Insinga F, Jimenez SA (1992) Growth properties and biochemical characterization of collagens synthesized by adult rat heart fibroblasts in culture. J Mol Cell Cardiol 24(7):691–700
Nagase H, Woessner JF Jr (1999) Matrix metalloproteinases. J Biol Chem 30;274(31):21491–21494
Spinale FG, Coker ML, Heung LJ, Bond BR, Gunasinghe HR, Etoh T, Goldberg AT, Zellner JL, Crumbley AJ (2000) A matrix metalloproteinase induction/activation system exists in the human left ventricular myocardium and is upregulated in heart failure. Circulation 17;102(16):1944–1949
Thomas CV, Coker ML, Zellner JL, Handy JR, Crumbley AJ III, Spinale FG (1998) Increased matrix metalloproteinase activity and selective upregulation in LV myocardium from patients with end-stage dilated cardiomyopathy. Circulation 5;97(17):1708–1715
Ducharme A, Frantz S, Aikawa M, Rabkin E, Lindsey M, Rohde LE, Schoen FJ, Kelly RA, Werb Z, Libby P, Lee RT (2000) Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest 106(1):55–62
Spinale FG, Coker ML, Bond BR, Zellner JL (2000) Myocardial matrix degradation and metalloproteinase activation in the failing heart: a potential therapeutic target. Cardiovasc Res 46(2):225–238
Seccia TM, Bettini E, Vulpis V, Quartaroli M, Trist DG, Gaviraghi G, Pirrelli A (1999) Extracellular matrix gene expression in the left ventricular tissue of spontaneously hypertensive rats. Blood Press 8(1):57–64
Acknowledgments
This study was supported in part by NIH RO-1 HL27738 and HL 47794 granted to S. Sen. Author wishes to thank Mr. David Young for his skilled technical assistance. We thankfully acknowledge heart transplant team, Dr. Christine Moravec and Wendy Sweet (Transplant Tissue Core, Cleveland Clinic, Cleveland, Ohio) for providing the human hearts. The author also wishes to thank Ms. Lori Sims for her excellent secretarial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
P. Sivakumar and Sudhiranjan Gupta contributed equally to this work.
Electronic supplementary material
Below are the electronic supplementary materials.
Rights and permissions
About this article
Cite this article
Sivakumar, P., Gupta, S., Sarkar, S. et al. Upregulation of lysyl oxidase and MMPs during cardiac remodeling in human dilated cardiomyopathy. Mol Cell Biochem 307, 159–167 (2008). https://doi.org/10.1007/s11010-007-9595-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-007-9595-2