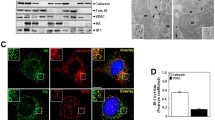
Abstract
The creatine kinase (CK) system is essential for cellular energetics in tissues or cells with high and fluctuating energy requirements. Creatine itself is known to protect cells from stress-induced injury. By using an siRNA approach to silence the CK isoenzymes in human keratinocyte HaCaT cells, expressing low levels of cytoplasmic CK and high levels of mitochondrial CK, as well as HeLa cancer cells, expressing high levels of cytoplasmic CK and low levels of mitochondrial CK, we successfully lowered the respective CK expression levels and studied the effects of either abolishing cytosolic brain-type BB-CK or ubiquitous mitochondrial uMi-CK in these cells. In both cell lines, targeting the dominant CK isoform by the respective siRNAs had the strongest effect on overall CK activity. However, irrespective of the expression level in both cell lines, inhibition of the mitochondrial CK isoform generally caused the strongest decline in cell viability and cell proliferation. These findings are congruent with electron microscopic data showing substantial alteration of mitochondrial morphology as well as mitochondrial membrane topology after targeting uMi-CK in both cell lines. Only for the rate of apoptosis, it was the least expressed CK present in each of the cell lines whose inhibition led to the highest proportion of apoptotic cells, i.e., downregulation of uMi-CK in case of HeLaS3 and BB-CK in case of HaCaT cells. We conclude from these data that a major phenotype is linked to reduction of mitochondrial CK alone or in combination with cytosolic CK, and that this effect is independent of the relative expression levels of Mi-CK in the cell type considered. The mitochondrial CK isoform appears to play the most crucial role in maintaining cell viability by stabilizing contact sites between inner and outer mitochondrial membranes and maintaining local metabolite channeling, thus avoiding transition pore opening which eventually results in activation of caspase cell-death pathways.



Similar content being viewed by others
References
Wallimann T, Wyss M, Brdiczka D et al (1992) Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem J 281(Pt 1):21–40
Wallimann T, Tokarska-Schlattner M, Neumann D et al (2007) The phospho-creatine circuit: molecular and cellular physiology of creatine kinases sensitivity to free radicals and enhancement by creatine supplementation. In: Saks V (ed) Molecular systems bioenergetics: basic principles, organization and dynamics of cellular energetics. Wiley Publisher Co., USA
Dolder M, Walzel B, Speer O et al (2003) Inhibition of the mitochondrial permeability transition by creatine kinase substrates. Requirement for microcompartmentation. J Biol Chem 278:17760–17766
Schlattner U, Tokarska-Schlattner M, Wallimann T (2006) Mitochondrial creatine kinase in human health and disease. Biochim Biophys Acta 1762:164–180
Wallimann T, Hemmer W (1994) Creatine kinase in non-muscle tissues and cells. Mol Cell Biochem 133–134:193–220
Kaldis P, Kamp T, Piendl T et al (1997) Functions of creatine kinase isoenzymes in spermatozoa. Adv Dev Biochem 5:275–312
Shin JB, Streijger F, Beynon A et al (2007) Hair bundles are specialized for ATP delivery via creatine kinase. Neuron 53:371–386
Schlattner U, Mockli N, Speer O et al (2002) Creatine kinase and creatine transporter in normal, wounded, and diseased skin. J Invest Dermatol 118:416–423
Lenz H, Schmidt M, Welge V et al (2005) The Creatine kinase system in human skin: protective effects of creatine against oxidative and UV damage in vitro and in vivo. J Invest Dermatol 124(2):443–452
Schulze A (2003) Creatine deficiency syndromes. Mol Cell Biochem 244:143–150
Brewer GJ, Wallimann TW (2000) Protective effect of the energy precursor creatine against toxicity of glutamate and beta-amyloid in rat hippocampal neurons. J Neurochem 74:1968–1978
Sullivan PG, Geiger JD, Mattson MP et al (2000) Dietary supplement creatine protects against traumatic brain injury. Ann Neurol 48:723–729
Klivenyi P, Ferrante RJ, Matthews RT et al (1999) Neuroprotective effects of creatine in a transgenic animal model of amyotrophic lateral sclerosis. Nat Med 5:347–350
Matthews RT, Ferrante RJ, Klivenyi P et al (1999) Creatine and cyclocreatine attenuate MPTP neurotoxicity. Exp Neurol 157:142–149
Ferrante RJ, Andreassen OA, Jenkins BG et al (2000) Neuroprotective effects of creatine in a transgenic mouse model of Huntington’s disease. J Neurosci 20:4389–4397
Zhu S, Li M, Figueroa BE et al (2004) Prophylactic creatine administration mediates neuroprotection in cerebral ischemia in mice. J Neuroscience 24:5909–5912
Hatano E, Tanaka A, Kanazawa A et al (2004) Inhibition of tumor necrosis factor-induced apoptosis in transgenic mouse liver expressing creatine kinase. Liver Int 24:384–393
Vandenberghe K, Goris M, Van Hecke P et al (1997) Long-term creatine intake is beneficial to muscle performance during resistance training. J Appl Physiol 83:2055–2063
Beutner G, Ruck A, Riede B et al (1998) Complexes between porin, hexokinase, mitochondrial creatine kinase and adenylate translocator display properties of the permeability transition pore. Implication for regulation of permeability transition by the kinases. Biochim Biophys Acta 1368:7–18
Meyer LE, Machado LB, Santiago AP et al (2006) Mitochondrial creatine kinase activity prevents reactive oxygen species generation: antioxidant role of mitochondrial kinase-dependent ADP re-cycling activity. J Biol Chem 281:37361–37371
Kay L, Nicolay K, Wieringa B et al (2000) Direct evidence for the control of mitochondrial respiration by mitochondrial creatine kinase in oxidative muscle cells in situ. J Biol Chem 275:6937–6944
van Deursen J, Wieringa B (1994) Approaching the multifaceted nature of energy metabolism: inactivation of the cytosolic creatine kinases via homologous recombination in mouse embryonic stem cells. Mol Cell Biochem 133–134:263–274
Steeghs K, Oerlemans F, de Haan A et al (1998) Cytoarchitectural and metabolic adaptations in muscles with mitochondrial and cytosolic creatine kinase deficiencies. Mol Cell Biochem 184:183–194
de Groof AJ, Smeets B, Groot Koerkamp MJ et al (2001) Changes in mRNA expression profile underlie phenotypic adaptations in creatine kinase-deficient muscles. FEBS Lett 506:73–78
de Groof AJ, Oerlemans FT, Jost CR et al (2001) Changes in glycolytic network and mitochondrial design in creatine kinase-deficient muscles. Muscle Nerve 24:1188–1196
Jost CR, Van Der Zee CE, In ‘t Zandt HJ et al (2002) Creatine kinase B-driven energy transfer in the brain is important for habituation and spatial learning behaviour, mossy fibre field size and determination of seizure susceptibility. Eur J Neurosci 15:1692–1706
In ‘t Zandt HJ, Renema WK, Streijger F et al (2004) Cerebral creatine kinase deficiency influences metabolite levels and morphology in the mouse brain: a quantitative in vivo 1H and 31P magnetic resonance study. J Neurochem 90:1321–1330
Momken I, Lechene P, Koulmann N et al (2005) Impaired voluntary running capacity of creatine kinase-deficient mice. J Physiol 565:951–964
Streijger F, Oerlemans F, Ellenbroek BA et al (2005) Structural and behavioural consequences of double deficiency for creatine kinases BCK and UbCKmit. Behav Brain Res 157:219–234
Novotova M, Pavlovicova M, Veksler VI et al (2006) Ultrastructural remodeling of fast skeletal muscle fibers induced by invalidation of creatine kinase. Am J Physiol Cell Physiol 291:C1279–C1285
Spindler M, Meyer K, Stromer H et al (2004) Creatine kinase-deficient hearts exhibit increased susceptibility to ischemia-reperfusion injury and impaired calcium homeostasis. Am J Physiol Heart Circ Physiol 287:H1039–H1045
Speer O, Back N, Buerklen T et al (2005) Octameric mitochondrial creatine kinase induces and stabilizes contact sites between the inner and outer membrane. Biochem J 385:445–450
Ito S, Karnovsky JM (1968) Formaldehyde-glutaraldehyde-trinitrofixation (Yellow fix). J Cell Biol 39:168a–169a
Dzeja PP, Terzic A (2003) Phosphotransfer networks and cellular energetics. J Exp Biol 206:2039–2047
Miller EE, Evans AE, Cohn M (1993) Inhibition of rate of tumor growth by creatine and cyclocreatine. Proc Natl Acad Sci USA 90:3304–3308
O’Gorman E, Beutner G, Dolder M et al (1997) The role of creatine kinase in inhibition of mitochondrial permeability transition. FEBS Lett 414:253–257
Kroemer G, Galluzzi L, Brenner C (2007) Mitochondrial membrane permeabilization in cell death. Physiol Rev 87:99–163
Wallimann T, Dolder M, Schlattner U et al (1998) Some new aspects of creatine kinase (CK): compartmentation, structure, function and regulation for cellular and mitochondrial bioenergetics and physiology. Biofactors 8:229–234
Kreider RB (2003) Effects of creatine supplementation on performance and training adaptations. Mol Cell Biochem 244:89–94
Brosnan JT, Brosnan ME (2007) Creatine: endogenous metabolite, dietary, and therapeutic supplement. Annu Rev Nutr [Epup ahead of print]
Balsom PD, Soderlund K, Ekbolm B (1994) Creatine in humans with special reference to creatine supplementation. Sports Med 18:268–280
Wyss M, Wallimann T (1994) Creatine metabolism and the consequences of creatine depletion in muscle. Mol Cell Biochem 133–134:51–66
Guerrero-Ontiveros ML, Wallimann T (1998) Creatine supplementation in health and disease. Effects of chronic creatine ingestion in vivo: down-regulation of the expression of creatine transporter isoforms in skeletal muscle. Mol Cell Biochem 184:427–437
Beal MF (2003) Bioenergetic approaches for neuroprotection in Parkinson’s disease. Ann Neurol 53 Suppl 3:S39–S47; discussion S47–8
Andres RH, Ducray AD, Huber AW et al (2005) Effects of creatine treatment on survival and differentiation of GABA-ergic neurons in cultured striatal tissue. J Neurochem 95(1):33–45
Andres RH, Huber AW, Schlattner U et al (2005) Effects of creatine treatment on the survival of dopaminergic neurons in cultured fetal ventral mesencephalic tissue. Neuroscience 133:701–713
Hausmann ON, Fouad K, Wallimann T, et al (2002) Protective effects of oral creatine supplementation on spinal cord injury in rats. Spinal Cord 40:449–456
Berneburg M, Gremmel T, Kurten V et al (2005) Creatine supplementation normalizes mutagenesis of mitochondrial DNA as well as functional consequences. J Invest Dermatol 125:213–220
Zemtsov A (2007) Skin phosphocreatine. Skin Res Technol 13:115–118
Acknowledgments
We thank Dr. Gesa Muhr and Dr. Ute Breitenbach, Beiersdorf AG (Hamburg, Germany) for support of the siRNA transfection experiments. Further we thank Ursula Lehr, Brigitte Agricola, and Volkwin Kramer, Department of Cytobiology and Cytopathology (Philipps University, Marburg) for their assistance with the transmission electron microscopy and photography. This work was supported by a grant from the Swiss National Science Foundation No. 31000AO-102075 (to T.W. and U.S.) and a “Chaire d’excellence” (ANR France, to U.S.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lenz, H., Schmidt, M., Welge, V. et al. Inhibition of cytosolic and mitochondrial creatine kinase by siRNA in HaCaT- and HeLaS3-cells affects cell viability and mitochondrial morphology. Mol Cell Biochem 306, 153–162 (2007). https://doi.org/10.1007/s11010-007-9565-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-007-9565-8