Abstract
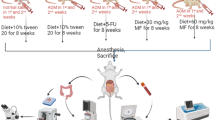
The present study evaluated the inhibitory effects of zinc on colonic antioxidant defense system and histoarchitecture during 1,2 dimethylhydrazine (DMH) induced colon carcinogenesis in male Sparque Dawley rats. The rats were segregated into four groups viz., normal control, DMH treated, zinc treated, DMH + zinc treated. Colon carcinogenesis was induced through weekly subcutaneous injections of DMH (30 mg/kg body weight) for 8 weeks. Zinc (in the form of zinc sulphate) was supplemented to rats at a dose level of 227 mg/l in drinking water, ad libitum for the entire duration of the study. Increased lipid peroxidation was accompanied by a decrease in reduced glutathione (GSH), glutathione reductase (GR), glutathione-s-transferase (GST), superoxide dismutase (SOD), and catalase. Administration of zinc to DMH treated rats significantly decreased the lipid peroxidation levels with simultaneous enhancement of GSH, GR, GST, SOD, and Catalase. Histopathological studies from DMH treated rats revealed disorganization of colonic histoarchitecture. However, zinc treatment to DMH treated rats greatly restored normalcy in the colonic histoarchitecture, with no apparent signs of abnormality. Energy Dispersive X-Ray Fluorescence (EDXRF) studies revealed a significant decrease in tissue concentrations of zinc in the colon following DMH treatment, which upon zinc supplementation were recovered to near normal levels. In conclusion, the results of this study suggest that zinc has a beneficial effect during the initiation of key events leading to the development of experimentally induced carcinogenesis.




Similar content being viewed by others
References
Shike M, Winawer SJ, Greenwald PH, Bloch A, Hill MJ, Swaroop SV (1990) Primary prevention of colorectal cancer:the WHO collaborating centre for prevention of colorectal cancer. Bull World Health Org 68:377–385
Pitot HC (1986) Fundamentals of oncology. Marcel Dekker, Inc., New York
Bartsch H, Nair J (2002) Potential role of lipid peroxidation derived DNA damage in human colon carcinogenesis: studies on exocyclic base adducts as stable oxidative stress markers. Cancer Detect Preven 22:308–312
Rogers AE, Nauss KM (1985) Rodent models for carcinoma of the colon. Dig Dis Sci 30:87S-102S
Manju V, Nalini N (2005) Chemopreventive efficacy of ginger, a naturally occurring anticarcinogen during the initiation, post-initiation stages of 1,2 dimethylhydrazine-induced colon cancer. Clin Chim Acta 58:60–67
Potter JD, McMichael AJ (1986) Diet and cancer of the colon and rectum: a case-control study. J Natl Cancer Inst 76:557–569
Mukhtar H, Athar M (1988) Dietary anticarcinogens and cancer prevention. Clevel Clin J Med 55:507–508
Carter JW, Lancaster H, Hardman WE, Cameron IL (1997) Zinc deprivation promotes progression of 1,2-dimethylhydrazine-induced colon tumors but reduces malignant invasion in mice. Nutr Cancer 27(3):217–221
Singh KP, Zaidi SIA, Raisuddin S, Saxena AK, Murthy RC, Ray PK (1992) Effect of zinc on immune functions and host resistance against infection and tumor challenge. Immunopharmacol Immunotoxicol 14:813–840
Satoh M, Kondo Y, Mita M, Nakagawa I, Naganuma A, Imura N (1993) Prevention of carcinogenecity of anticancer drugs by metallothionein induction. Cancer Res 53:4767–4768
Soler AP, Miller RD, Laughlin KV, Carp NZ, Klurfeld DM, Mullin JM (1999) Increased tight junctional permeability is associated with the development of colon cancer. Carcinogenesis 20:1425–1431
Goel A, Dani V, Dhawan DK (2005) Protective effects of zinc on lipid peroxidation, antioxidant enzymes and hepatic histoarchitecture in chlorpyrifos-induced toxicity. Chem Biol Interact 156:131–140
Wills ED (1966) Mechanism of lipid peroxide formation in animal tissues. Biochem J 99:667–676
Ellman GL (1959) Tissue sulphydryl groups. Arch Biochem Biophys 82:70–77
Luck H (1971) In: Bergmeyer HU (ed) Methods of enzymatic analysis. Academic Press, New York, pp 885–893
Kono Y (1978) Generation of superoxide radical during auto oxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys 186:189–195
Carlberg I, Mannervik B (1985) Glutathione reductase. Methods Enzymol 113:484–490
Habig WH, Pabst MJ, Jacoby WB (1974) Glutathione-S-Transferase: the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Lowry OH, Rorebrough NJ, Farr AL, Randall J (1951) Protein measurement with the folin phenol reagent. J Biol Chem 93:265–275
Dudeja PK, Brasitus TA (1990) 1,2-Dimethylhydrazine-induced alterations in lipid peroxidation in preneoplastic and neoplastic colonic tissues. Biochim Biophys Acta 1046:267–270
Hendrickse CW, Kelly R, Radley S, Donovan IA, Keighley MR, Neoptolemod JP (1994) Lipid peroxidation and prostaglandins in colorectal cancer. Br J Surg 81:1219–1223
Skrzydlewska E, Stanklewicz A, Michalak K, Sulkowaska M, Zalewski B, Piotrowski Z (2001) Antioxidant status and proteolytic-antiproteolytic balance in colorectal cancer. Folia Histochem Cytobiol 39:98–99
Seven A, Civelek S, Inci E, Korkut N, Burçak G (1999) Evaluation of oxidative stress parameters in blood of patients with laryngeal carcinoma. Clin Biochem 32:369–373
Chvapil M, Ryan JN, Zukoski CF (1972) Effect of zinc on lipid peroxidation in liver microsomes and mitochondria. Proc Soc Exp Biol Med 140:642–646
Cabre M, Ferre N, Folch J, Paternain JL, Hernandez M, Castillo D, Joven G, Camps J (1995) Inhibition of hepatic cell nuclear DNA fragmentation by zinc in carbon tetrachloride-treated rats. J Hepatol 31:228–234
Bettger WJ, O’Dell BL (1981) A critical physiological role of zinc in the structure and function of biomembranes. Life Sci 28:1425–1438
Pardeep S, Garg ML, Dhawan DK (2004) Protective role of zinc in nickel induced hepatotoxicity in rats. Chem Biol Interact 15:199–209
Younes M, Siegers CP (1981) Mechanistic aspects of enhanced lipid peroxidation following glutathione dependent depletion in vivo. Chem Biol Interact 34:257–266
Jakoby WB (1978) The glutathione tranferase in detoxification. In: Sies H, Wendel S (Eds) Functions of glutathione in liver and kidney. Springer, Berlin, pp 157–163
Al-Turk WA, Stohs SJ, El-Rashidy FH, Othman S, Shaheen O (1987) Changes in the glutathione, glutathione reductase and glutathione-s-transferase as a function of cell concentration and age. Pharmacology 34:1–8
Ludwig JC, Misiorowski RL, Chvapil M, Seymour MD (1980) Interaction of zinc ions with electron carrying coenzymes NADPH and NADH. Chem Biol Interact 30:25–34
Kyle ME, Miccadie S, Nakae D, Farber JL (1987) Superoxide dismutase and catalase protect cultured hepatocytes from the cytotoxicity of acetaminophen. Biochem Biophys Res Commun 149:889–896
Mates JM, Sanchez-Jimenez F (1999) Antioxidant enzymes and their implications in pathophysiologic processes. Front Biosci 4:334–345
Burton GW, Cheesman KN, Ingold KV, Seater TF (1983) Lipid antioxidants and products of lipid peroxidation as potential tumor protective agents. Biochem Soc Trans 11:261–262
Reddy SB, John Charles M, Naga Raju GJ, Seetharami Reddy B, Seshi Reddy T, Rama Lakshmi PVB, Vijayan V (2004) Trace elemental analysis of cancer-afflicted intestine by PIXE technique. Biological Trace Element Res 102:265–281
Franklin RB, Feng P, Milon B, Desouki MM, Singh KK, Kajdaksy-Balla A, Bagasra O, Costello LC (2005) hZIP1 zinc uptake transporter down regulation and zinc depletion in prostate cancer. Mol Cancer 4:32
Szmigielski S, Litwin J (1964) The histochemical demonstration of zinc in blood granulocytes-the new test in diagnosis of neoplastic diseases. Cancer 17:1381–1384
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chadha, V.D., Vaiphei, K. & Dhawan, D.K. Zinc mediated normalization of histoarchitecture and antioxidant status offers protection against initiation of experimental carcinogenesis. Mol Cell Biochem 304, 101–108 (2007). https://doi.org/10.1007/s11010-007-9490-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-007-9490-x