Abstract
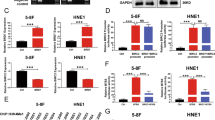
BRD7 is a novel gene which involved NPC in our lab. Our previous studies showed that BRD7 was expressed at high level in normal nasopharyngeal epithelial tissues, but at low level in nasopharyngeal carcinoma biopsies and cell lines. In these papers, we found that ectopic expression of BRD7 can decrease cell proliferation and capability to form colonies in soft agar. FCM (Flow cytometry) assay indicated that the cell cycle progression from G1 to S phase was inhibited and the expression of cyclinD1 was significantly decreased after being transfected with BRD7 in HNE1 cells (NPC cells). To further investigate the molecular mechanism of BRD7 suppression of NPC cells growth, the cDNA microarray was performed to detect difference in gene expression profile induced by BRD7. The results indicated that 21 genes expression were changed after being transfected with BRD7 and the differentially expressed gene including α-catenin, cyclinD1, E2F3 was confirmed by western-blot. Next, we found that even though no obvious changes of the total expression of β-catenin were observed, the accumulation of β-catenin in nucleus was blocked. In addition, it was found that the expression of β-catenin was up-regulated in the complex composed of β-catenin and α-catenin in HNE1 cells induction of BRD7. So, we concluded that over-expression of BRD7 increased the expression of α-catenin which “hold” β-catenin in the complex and inhibited its accumulating in nucleus. At last, we demonstrated the c-jun, p-MEK, and p-ERK1/2 expression were down-regulated, and the Ap-1 promoter activity was inactive after being transfected with BRD7. We also found that over-expression of BRD7 can inactivate the c-jun and p-ERK1/2 after being treated with EGF in HNE1 cells. These results indicated that BRD7 played a negative role in ERK1/2 pathway. Taken together, our present results provide new insights for BRD7 function to inhibit NPC cells growth through negative regulating β-catenin and ERK1/2 pathways.





Similar content being viewed by others
References
Lo RW, Tsao S-W, Leung S-F, Choi PHK, Lee JCK, Huang DP (1994) Detailed deletion mapping on the short arm of chromosome 3 in nasopharyngeal carcinoma. Int J Oncol 4:1359–1364
Hu LF, Eiriksdottir G, Lebedeva T, Kholodniouk I, Alimov A, Chen F, Luo Y, Zabarovsky ER, Ingvarsson S, Klein G, Ernberg I (1996) Loss of heterozygosity on chromosome arm 3p in nasopharyngeal carcinoma. Genes Chromosomes Cancer 17(2):118–126
Mutirangura A, Pornthanakasem W, Sriuranpong V, Supiyaphun P, Voravud N (1998) Loss of heterozygosity on chromosome 14 in nasopharyngeal carcinoma. Int J Cancer 78(2):153–156
Shao JY, Wang HY, Huang XM, Feng QS, Huang P, Feng BJ, Huang LX, Yu XJ, Li JT, Hu LF, Ernberg I, Zeng YX (2000) Genome-wide allelotype analysis of sporadic primary nasopharyngeal carcinoma from southern China. Int J Oncol 17(6):1267–1275
Hui AB, Lo KW, Leung SF, Teo P, Fung MK, To KF, Wong N, Choi PH, Lee JC, Huang DP (1999) Detection of recurrent chromosomal gains and losses in primary nasopharyngeal carcinoma by comparative genomic hybridisation. Int J Cancer. 82(4):498–503
Chen YJ, Ko JY, Chen PJ, Shu CH, Hsu MT, Tsai SF, Lin CH (1999) Chromosomal aberrations in nasopharyngeal carcinoma analysis by comparative genemic hybridization. Genes Chromosomes Cancer 25(2):169–175
Feng BJ, Huang W, Shugart YY, Lee MK, Zhang F, Xia JC, Zeng YX (2002) Genome-wide scan for familial nasopharygeal carcinoma reveals evidence of linkage to chromosome 4. Nat Genet 31:395–399
Xiong W, Zeng ZY, Xia JH, Xia K, Shen SR, Li XL, Li GY (2004) A susceptibility locus at chromosome 3p21 linked to familial nasopharygeal carcinoma. Cancer Res (15):1972–1974
Yu Y, Xie Y, Cao L, Zhang BC, Zhong M, Zhang FH, Li GY (2000) Isolation of tumor differentially expressed genes by mixing probes library screen. Chin J Cancer 19(7):709–712
Yu Y, Zhu SG, Zhang BC, Zhou M, Li XL, Li GY (2000) Coding-region single nucleotide polymorphisms in BRD7gene and nasopharyngeal carcinoma susceptibility. Prog Biochem Biophys 28(4):568–572
Yu Y, Zhu SG, Zhang BC, Zhou M, Li XL, Li GY (2002) Screening of BRD7 interacting proteins by yeast two-hybrid system. Sci China C 45(5):546–552
Peng C, Zhou J, Liu HY, Zhou M Li GY et al (2005) The transcriptional regulation role of BRD7 by binding to acetylated histone through bromodomain. J Cell Biochem 97(4):882–892
Kzhyshkowska J, Rusch A, Wolf H, Dobner T (2003) Regulation of transcription by the heterogeneous nuclear ribonucleoprotein E1B-Ap5 is mediated by complex formation with the novel bromodomain-containing protein BRD7. Biochem J 371:385–393
Staal A, Enserink JM, Stein JL, Stein GS, Van Wijnen AJ (2000) Molecular characterization of celtix-1, a bromodomain protein interacting with the transcription factor interferon regulatory factor 2. J Cell Physiol 185:269–279
Li JJ, Westergaard C, Ghosh P, Colburn NH (1997) Inhibitors of both nuclear factor-kappa B and activator protein-1 activation block the neoplastic transformation response. Cancer Res 57(16):3569–3576
Peng C, Liang SP, Tang C, Li GY et al (2003) Researching a novel NPC-related candidate suppressor gene BRD7 by two-dimensional gel electrophoresis and MALDI-TOF-MS. Acta Biochimica et Biophysica Sinica(ABBS) 35(9):816–822
Chen R, Kim O, Li M, Xiong X, Guan JL, Kung HJ, Chen H, Shimizu Y, Qiu Y (2001) Regulation of the PH-domain-containing tyrosine kinase Etk by focal adhesion kinase through the FERM domain. Nat Cell Biol 3:439–444
Zhou J, Ma J, Zhang BC, Li XL, Shen SR, Zhu SG, Xiong W, Liu HY, Huang H, Zhou M, Li GY (2004) BRD7, a novel bromodomain gene, inhibits G1-S progression by transcriptionally regulating some important molecules involved in ras/MEK/ERK and RB/E2F pathways. J Cell Physiol 200:89–98
Ben Ze’ev A (1999) The dual role of cytoskeletal anchor proteins in cell adhesion and signal transduction. Ann NY Acad Sci 886:37–47
Willert K, Nusse R (1998) Beta-Catenin: a key mediator of Wnt signaling. Curr Opin Genet Dev 8:95–102
Taipale J, Beachy PA (2001) The Hedgehog and Wnt signaling pathways in cancer. Nature 411:349–354
Novak A, Hsu SC, Leung-Hagesteijn C et al (1998) Cell adhesionand the integrin-linked kinase regulate the LEF-1 and betacateninsignaling pathways. Proc Natl Acad Sci 95:4374–4379
Delcommenne M, Tan C, Gray V et al (1998) Phosphoinositide-3-OH kinase-dependent regulation of glycogensynthase kinase 3 and protein kinase B/AKT by the integrin linked kinase. Proc Natl Acad Sci 95:11211–11216
Zheng Z, Pan J, Chu B, Wong YC, Cheung AL, Tsao SW (1999) Down-regulation and abnormal expression of E-cadherin and beta-catenin in nasopharyngeal carcinoma: close association with advanced disease stage and lymph node metastasis. Hum Pathol 30:458–66
Morrison JA, Gulley ML, Pathmanathan R, Raab-Traub N (2004) Differential signaling pathways are activated in the Epstein–Barr virus–associated malignancies nasopharyngeal carcinoma and Hodgkin lymphoma. Cancer Res 64:5251–5260
Zheng ZY, Zhou YH, Zhang WL, Xiong W, Fan SQ, Li XL, Li GY et al (2007) Gene expression profiling of nasopharyngeal carcinoma reveals the abnormally regulated Wnt signaling pathway. Hum Pathol 38:120–133
Hommes DW, Peppelenbosch MP, van Deventer SJH (2003) Mitogen activated protein (MAP) kinase signal transduction pathways and novel anti-inflammatory targets. Gut 52:144–151
Troppmair J, Bruder JT, Munoz H et al (1994) Mitogen-activated protein kinase extracellular signal-regulated protein kinase activation by oncogenes, serum, and 12-O-tetradecanoylphorbol-13-acetate requires Raf and is necessary for transformation. J Biol Chem 269:7030–7035
DeFazio A, Chiew YE, Sini RL et al (2000) Expression of cerbB receptors, heregulin and oestrogen receptor in human breast cell lines. Int J Cancer 87:487–498
Treisman R (1994) Ternary complex factors: growth factor regulated transcriptional activators. Curr Opin Genet Dev 4:96–101
Lewis TS, Shapiro PS, Ahn NG (1998) Signal transduction through MAP kinase cascades. Adv Cancer Res 74:49–139
Young MR, Li JJ, Rincon M, Flavell RA, Sathyanarayana BK, Hunziker R, Colburn N (1999) Transgenic mice demonstrate AP-1 (activator protein-1) transactivation is required for tumor promotion. Proc Natl Acad Sci USA 96:9827–9832
Lopez-Ilasaca M (1998) Signaling from G-protein-coupled receptors to mitogen-activated protein (MAP)-kinase cascades. Biochem Pharmacol 56(3):269–277
Peyssonnaux C, Eychene A (2001) The Raf/MEK/ERK pathway: New concepts of activation. Biol Cell 93(1–2):53–62
Yun MS, Kim SE, Jeon SH et al (2005) Both ERK and Wnt/β-catenin pathways are involved in Wnt3a-induced proliferation. J Cell Sci 118:313–322
Acknowledgments
We are grateful to Dr. J.J. Li for his kind provision of AP1 reporter plasmid luciferase reporter plasmid. This work is supported by grants from the National Natural Science Foundation of China (30400528; 30400238; 30470367.) and Hunan Natural Scientific Foundation of China (04JJ3097)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peng, C., Liu, H.Y., Zhou, M. et al. BRD7 suppresses the growth of Nasopharyngeal Carcinoma cells (HNE1) through negatively regulating β-catenin and ERK pathways. Mol Cell Biochem 303, 141–149 (2007). https://doi.org/10.1007/s11010-007-9466-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-007-9466-x