Abstract
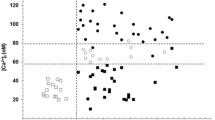
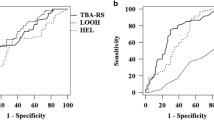
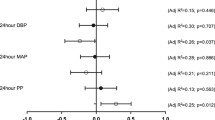
Oxidative stress may play a role in the pathogenic mechanism of essential hypertension. Lipid peroxidation can alter the cellular structure of membrane-bound enzymes by changing the membrane phospholipids fatty acids composition. We investigated the relationship between (Na + K)-ATPase activity, lipid peroxidation, and erythrocyte fatty acid composition in essential hypertension. The study included 40 essential hypertensive and 49 healthy normotensive men (ages 35–60 years). Exclusion criteria were obesity, dyslipidemia, diabetes mellitus, smoking, and any current medication. Patients underwent 24-h ambulatory blood pressure monitoring and blood sampling. Lipid peroxidation was measured in the plasma and erythrocytes as 8-isoprostane or malondialdehyde (MDA), respectively. Antioxidant capacity was measured as ferric reducing ability of plasma (FRAP) in the plasma and as reduced/oxidized glutathione (GSH/GSSG ratio) in erythrocytes. (Na + K)-ATPase activity and fatty acids were determined in erythrocyte membranes. Hypertensives had higher levels of plasma 8-isoprostane, erythrocyte MDA, and relative percentage of saturated membrane fatty acids, but lower plasma FRAP levels, erythrocyte GSH/GSSG ratio, (Na + K)-ATPase activity and relative percentage of unsaturated membrane fatty acids, compared with normotensives. Day-time systolic and diastolic blood pressures correlated positively with lipid peroxidation parameters, but negatively with (Na + K)-ATPase activity. These findings suggest that the modulation of (Na + K)-ATPase activity may be associated with changes in the fatty acid composition induced by oxidative stress and provide evidence of a role for this enzyme in the pathophysiology of essential hypertension.





Similar content being viewed by others
References
Lassègue B, Griendling K (2004) Reactive oxygen species in hypertension. An update. Am J Hypertens 17:852–860
Touyz RM, Schiffrin EL (2004) Reactive oxygen species in vascular biology: implications in hypertension. Histochem Cell Biol 122:339–352
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr et al (2003) Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension 289:2560–2572
Stidley CA, Hunt WC, Tentori F, Schmidt D, Rohrscheib M, Paine S et al (2006) Changing relationship of blood pressure with mortality over time among hemodialysis patients. J Am Soc Nephrol 17:513–520
Chen X, Touyz RM, Bae Park J, Schiffrin EL (2001) Antioxidant effects of vitamins C and E are associated with altered activation of vascular NADPH oxidase and superoxide dismutase in stroke-prone SHR. Hypertension 38:606–611
Pedro-Botet J, Covas MI, Martin S, Rubies-Prat J (2000) Decreased endogenous antioxidant enzymatic status in essential hypertension. J Hum Hypertens 14:343–345
John S, Schmieder RE (2003) Potential mechanisms of impaired endothelial function in arterial hypertension and hypercholesterolemia. Curr Hypertens Rep 5:199–207
Stark G (2005) Functional consequences of oxidative membrane damage. Membr Biol 205:1–16
Cazzola R, Rondanelli M, Russo-Volpe S, Ferrari E, Cestaro B (2004) Decreased membrane fluidity and altered susceptibility to peroxidation and lipid composition in overweight and obese female erythrocytes. J Lipid Res 45:1846–1851
Elizondo A, Araya J, Rodrigo R, Poniachik J, Signorini C, Sgherri C, Comporti M, Videla LA (2007) Docosahexaenoic acid and docosapentaenoic acid levels in liver and erythrocyte phospholipids from obese non-alcoholic fatty liver disease patients. Obesity 15:24–31
Felton CV, Stevenson JC, Godsland IF (2004) Erythrocyte-derived measures of membrane lipid composition in healthy men: associations with arachidonic acid at low to moderate not high insulin sensitivity. Metabolism 53:571–577
Vajreswari A, Rupalatha M, Rao PS (2002) Effect of altered dietary n-6-to-n-3 fatty acid ratio on erythrocyte lipid composition and membrane-bound enzymes. J Nutr Sci Vitaminol (Tokio) 48:365–370
Bartoli GM, Palozza P, Luberto C, Franceschelli P, Piccioni E (1995) Dietary fish oil inhibits human erythrocyte Mg,NaK-ATPase. Biochem Biophys Res Commun 213:881–887
Rodrigo R, Castillo R (2006) Pathophysiological mechanisms of renal damage by alcohol consumption: involvement of oxidative stress. In: Yoshida R (ed) Trends in Alcohol Abuse and Alcoholism Research. Nova Publishers
Grover AK, Samson SE, Robinson S, Kwan CY (2003) Effects of peroxynitrite on sarcoplasmic reticulum Ca2+ pump in pig coronary artery smooth muscle. Am J Physiol Cell Physiol 284:C294–C301
Hamilton BP, Blaustein MP (2006) Molecular mechanisms linking sodium to hypertension: report of a symposium. J Investig Med 54:86–94
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr et al (2003). Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension 289:2560–2572
Myers MG, Tobe SW, McKay DW, Bolli P (2005) New algorithm for the diagnosis of hypertension. Am J Hypertens 18:1369–1374
O’Brien E, Mee F, Atkins N, O’Malley K (1991) Short report: accuracy of the Spacelabs 90207 determined by to the British Hypertension Society Protocol. J Hypertens 9:573–574
Groppelli A, Omboni S, Parati G, Mancia G (1992) Evaluation of noninvasive blood pressure monitoring devices Spacelabs 90202 and 90207 versus resting and ambulatory 24-h intra-arterial blood pressure. Hypertension 20:227–232
Sun M, Tien J, Jones R, Ward R (1996) A new approach to reproducibility assessment: clinical evaluation of SpaceLabs Medical oscillometric blood pressure monitor. Biomed Instrum Technol 30:439–448
Benzie IF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239:70–76
Hissin PJ, Hilf R (1976) A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem 74:214–226
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Pradelles P, Grassi J, Maclouf J (1985) Enzyme immunoassays of eicosanoids using AchE as label: an alternative to radioimmunoassay. Anal Chem 57:1170–1173
Katz AI, Epstein FH (1967) The role of sodium–potassium-activated adenosine triphosphatase in the reabsorption of sodium by the kidney. J Clin Invest 46:1999–2011
Taussky HH, Shorr E (1953) A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem 202:675–685
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Huertas JR, Palomino N, Ochoa JJ, Quiles JL, Ramirez-Tortosa MC, Battino M, Robles R, Mataix J (1998) Lipid peroxidation and antioxidants in erythrocyte membranes of full-term and preterm newborns. Biofactors 8:133–137
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction. Can J Biochem Physiol 37:911–917
Araya J, Rodrigo R, Orellana M, Rivera G (2001) Red wine raises HDL and preserves long-chain polyunsaturated fatty acids in rat kidney and erythrocytes. Br J Nutr 86:189–195
Redon J, Oliva MR, Tormos C, Giner V, Chaves J, Iradi A, Saez GT (2003) Antioxidant activities and oxidative stress byproducts in human hypertension. Hypertension 41:1096–1101
Kashyap MK, Yadav V, Sherawat BS, Jain S, Kumari S, Khullar M et al (2005) Different antioxidants status, total antioxidant power and free radicals in essential hypertension. Mol Cell Biochem 277:89–99
Hozawa A, Ebihara S, Ohmori K, Kuriyama S, Ugajin T, Yayoi Koizumi Y et al (2004) Increased plasma 8-isoprostane levels in hypertensive subjects: the Tsurugaya project. Hypertens Res 27:557–561
Gibson RA, Neumann MA, Burnard SL, Rinaldi JA, Patten GS, McMurchie EJ (1992) The effect of dietary supplementation with eicosapentaenoic acid on the phospholipid and fatty acid composition of erythrocytes of marmoset. Lipids 27:169–176
Knapp HR, Hullin F, Salem N Jr (1994) Asymmetric incorporation of dietary n−3 fatty acids into membrane aminophospholipids of human erythrocytes. J Lipid Res 35:1283–1291
Alexander-North LS, North JA, Kiminyo KP, Buettner GR, Spector AA (1994) Polyunsaturated fatty acids increase lipid radical formation induced by oxidant stress in endothelial cells. J Lipid Res 35:1773–1785
Van Ginkel G, Sevanian A (1994) Lipid peroxidation-induced membrane structural alterations. Methods Enzymol 233:273–288
Leclerc L, Marden M, Poyart C (1991) Inhibition of the erythrocyte (Ca2++Mg2+)-ATPase by nonheme iron. Biochim Biophys Acta 1062:35–38
Fukuda K, Davies SS, Nakajima T, Ong BH et al (2005) Oxidative mediated lipid peroxidation recapitulates proarrhythmic effects on cardiac sodium channels. Circ Res 97:1262–1269
Kocak-Toker N, Giris M, Tulubas F, Uysal M, Aykac-Toker G (2005) Peroxynitrite induced decrease in Na+, K+-ATPase activity is restored by taurine. World J Gastroenterol 11:3554–3557
Norman RI, Achall N. (1993) Cell membrane microviscosity and Ca(2+)-Mg(2+)-ATPase activity do not contribute to hypertension in the spontaneously hypertensive rat model. Clin Sci (Lond) 85:585–591
Liu Y, Longmore RB (1997) Dietary sandalwood seed oil modifies fatty acid composition of mouse adipose tissue, brain, and liver. Lipids 32:965–969
Begin ME (1990) Fatty acids, lipid peroxidation and diseases. Proc Nutr Soc 49:261–267
De Lorgeril M, Salen P (2003) Dietary prevention of coronary heart disease: focus on omega-6/omega-3 essential fatty acid balance. World Rev Nutr Diet 92:57–73
Harris WS (2006) The omega-6/omega-3 ratio and cardiovascular disease risk: uses and abuses. Curr Atheroscler Rep 8:453–459
Wijendran V, Hayes KC (2004) Dietary n−6 and n−3 fatty acid balance and cardiovascular health. Annu Rev Nutr 24:597–615
Iwamoto T, Kita S (2006) Topics on the Na+/Ca2+ exchanger: role of vascular NCX1 in salt-dependent hypertension. J Pharmacol Sci 102:32–36
Hallaq HA, Haupert GT Jr (1989) Positive inotropic effects of the endogenous Na+/K(+)-transporting ATPase inhibitor from the hypothalamus. Proc Natl Acad Sci USA 86:10080–10084
Shaikh IM, Lau BW, Siegfried BA, Valdes R Jr (1991) Isolation of digoxin-like immunoreactive factors from mammalian adrenal cortex. J Biol Chem 266:13672–13678
Manunta P, Stella P, Rivera R et al (1999) Left ventricular mass, stroke volume, and ouabain-like factor in essential hypertension. Hypertension. 34:450–456
Zhang W, Huang BS, Leenen FH (1999) Brain renin-angiotensin system and sympathetic hyperactivity in rats after myocardial infarction. Am J Physiol 276:H1608–H1615
Dostanic-Larson I, Van Huysse JW, Lorenz JN, Lingrel JB (2005) The highly conserved cardiac glycoside binding site of Na,K-ATPase plays a role in blood pressure regulation. Proc Natl Acad Sci USA 102:15845–15850
Dostanic I, Paul RJ, Lorenz JN, Theriault S, Van Huysse JW, Lingrel JB (2005) The alpha2-isoform of Na-K-ATPase mediates ouabain-induced hypertension in mice and increased vascular contractility in vitro. Am J Physiol Heart Circ Physiol 288:H477–H485
Kaplan JH (2005) The sodium pump and hypertension: a physiological role for the cardiac glycoside binding site of the Na,K-ATPase. Proc Natl Acad Sci USA 102:15723–15724
Bova S, Blaustein MP, Ludens JH, Harris DW, DuCharme DW, Hamlyn JM (1991) Effects of an endogenous ouabain-like compound on heart and aorta. Hypertension 17:944–950
Acknowledgments
The authors wish to thank the Fondo Nacional de Investigación Científica y Tecnológica (FONDECYT, grant number 1040429, Chile Government), Procaps Laboratory (Colombia) and Gynopharm CFR Laboratory (Chile) for their financial support of this study. The technical assistance of Mr. Diego Soto is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodrigo, R., Bächler, J.P., Araya, J. et al. Relationship between (Na + K)-ATPase activity, lipid peroxidation and fatty acid profile in erythrocytes of hypertensive and normotensive subjects. Mol Cell Biochem 303, 73–81 (2007). https://doi.org/10.1007/s11010-007-9457-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-007-9457-y