Abstract
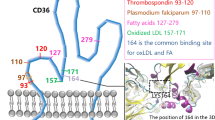
Oxidized low density lipoprotein (LDL) (Ox-LDL) plays an important role in the pathogenesis of atherosclerosis. Oxidized LDL is taken up by macrophages via scavenger receptors. CD36 is an 88 kDa glycoprotein expressed on platelets, monocyte-macrophages, microvascular endothelial cells, adipose tissue, skeletal muscles and heart. We found patients with CD36 deficiency and identified several mutations in the CD36 gene. We also reported that CD36-deficient macrophages showed a 50% reduction in the binding of Ox-LDL, suggesting that CD36 is one of the major receptors for Ox-LDL. CD36 was expressed on macrophages in the atherosclerotic lesions of human aorta and coronary arteries especially on foamed macrophages. The distribution of CD36 expression was slightly different from that of scavenger receptor class A types I and II. The expression of CD36 on macrophages was up-regulated by Ox-LDL and down-regulated by interferon γ. Since CD36 is a transporter of long-chain fatty acids (LCFA), CD36-deficient patients showed a defect in the uptake of an LCFA analog, BMIPP, by the heart. Furthermore, the secretion of IL-1β and TNF-α from monocyte-derived macrophages induced by Ox-LDL was markedly reduced and the activation of NF-κB was attenuated in CD36-deficient subjects compared with controls, suggesting that CD36-mediated signaling is also impaired in CD36 deficiency.
To elucidate the roles of CD36 in vivo, we characterized the clinical profile of CD36-deficient patients. Most of them were accompanied by hyperlipidemia (mainly hypertriglyceridemia), increased remnant lipoproteins and mild elevation of fasting plasma glucose level and blood pressure. Glucose clamp technique revealed mean whole body glucose uptake was reduced in CD36-deficient patients, indicating the presence of insulin resistance. The frequency of CD36 deficiency was higher in patients with coronary heart disease (CHD) than in control subjects. Taken together, CD36 deficiency is accompanied by (1) hyperlipidemia and increased remnant lipoproteins, (2) impaired glucose metabolism based upon insulin resistance, and (3) mild hypertension, and comprises one of the genetic backgrounds of the metabolic syndrome, leading to the development of CHD. (Mol Cell Biochem xxx: 1–4, 2004)
Similar content being viewed by others
References
Tandon NN, Lipsky RH, Burgess WH, et al: Protein isolation and characterization of platelet glycoprotein IV (CD36). J Biol Chem 264: 7570–7575, 1989.
Endemann G, Stanton IW, Madden KS, et al: CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem 268: 11811–11816, 1993.
Nozaki S, Kashiwagi H, Yamashita S, et al: Reduced uptake of oxidized low density lipoproteins in monocyte-derived macrophages from CD36-deficient subjects. J Clin Invest 96: 1859–1865, 1995.
Nagy L, Tontonoz P, Alvarez JG, et al: Oxidized LDL regulates macrophage gene expression through ligand activation of PPARγ. Cell 93: 229–240, 1998.
Nakagawa T, Nozaki S, Nishida M, et al: Oxidized LDL increases and interferon-gamma decreases expression of CD36 in human monocyte-derived macrophages. Arterioscler Thromb Vasc Biol 18: 1350–1357, 1998.
Nakata A, Nakagawa Y, Nishida M, et al: CD36, a novel receptor for oxidized low-density lipoproteins is highly expressed on lipid-laden macrophages in human atherosclerotic aorta. Arterioscler Thromb Vasc Biol 19: 1333–1139, 1999.
Nakagawa Y, Yamashita S, Nozaki S, et al: Differential expression of CD36 and scavenger receptor class A type I and type II in human coronary arteries. Atherosclerosis 156: 297–305, 2001.
Febbraio M, Hajjar DP, Silverstein RI: CD36, a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation and lipid metabolism. J Clin Invest 108: 785–791, 2001.
Yamamoto N, Ikeda H, Tandon NN, et al: A platelet membrane glycoprotein (GP) deficiency in healthy blood donors: Naka platelet lack detectable GPIV(CD36). Blood 76: 1698–1703, 1990.
Kashiwagi H, Honda S, Tomiyama Y, et al: A novel polymorphism in glycoprotein IV (replacement of proline-90 by serine) predominates in subjects with platelet GPIV deficiency. Thromb Haemost 69: 481–484, 1993.
Kashiwagi H, Tomiyama Y, Nozaki S, et al: Analyses of genetic abnormalities in type I CD36 deficiency in Japan: identification and cell biological characterization of two novel mutations that cause CD36 deficiency in man. Hum Genet 108: 459–466, 2001.
Janabi MY, Yamashita S, Hirano K, et al: Reduced adhesion of monocyte-derived macrophages from CD36-deficient patients to type I collagen. Biochem Biophys Res Commun 283: 26–30, 2001.
Janabi M, Yamashita S, Hirano K, et al: Oxidized low density lipoprotein-induced activation of NF-κB and subsequent expression of a variety of proinflammatory and proatherogenic genes are defective in monocyte-derived macrophages from CD36-deficient patients. Arterioscler Thromb Vasc Biol 20: 1953–1960, 2000.
Abumrad NA, el-Maghrabi MR, Amri EZ, et al: Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J Biol Chem 268: 17665–17668, 1993.
Nozaki S, Tanaka T, Yamashita S, et al: CD36 mediates long-chain fatty acid transport in human myocardium: complete myocardial accumulation defect of radiolabeled long-chain fatty acid analog in subjects with CD36 deficiency. Mol Cell Biochem 192: 129–135, 1999.
Tanaka T, Sohmiya K, Kawamura K: Is CD36 deficiency an etiology of hereditary hypertrophic cardiomyopathy? J Mol Cell Cardiol 29: 121–127, 1997.
Tanaka T, Nakata T, Oka T, et al: Defect in human myocardial long-chain fatty acid uptake is caused by FAT/CD36 mutation. J Lipid Res 42: 751–759, 2001.
Miyaoka K, Kuwasako T, Hirano K, et al: CD36 deficiency is associated with insulin resistance. Lancet 357: 686–687, 2001.
Yanai H, Chiba H, Morimoto M, et al: Human CD36 deficiency is associated with elevation in low-density lipoprotein-cholesterol. Am J Med Genet 93: 299–304, 2000.
Yanai H, Chiba H, Fujiwara H, et al: Metabolic changes in human CD36 deficiency displayed by glucose loading. Thromb Haemost 86: 995–999, 2001.
Kuwasako T, Hirano K, Sakai N, Ishigami M, Hiraoka H, Yakub MJ, Yamauchi-Takihara K, Yamashita S, Matsuzawa Y: Lipoprotein abnormalities in human genetic CD36 deficiency associated with insulin resistance and abnormal fatty acid metabolism. Diabetes Care 26: 1647–1648, 2003.
Yoshizumi T, Nozaki S, Fukuchi K, et al: Pharmacokinetics and metabolism of 123I-BMIPP fatty acid analog in healthy and CD36-deficient subjects. J Nucl Med 41: 1134–1138, 2000.
Matsumoto K, Hirano K, Nozaki S, et al: Expression of macrophage scavenger receptor, CD36 in cultured human aortic smooth muscle cells, in association with the expression of peroxisome proliferators activated receptor-γ. Arterioscler Thromb Vasc Biol 20: l027–l032, 2000.
Aitman TJ, Glazier AM, Wallace CA, et al: Identification of Cd36 (Fat) as an insulin-resistance gene causing defective fatty acid and glucose metabolism in hypertensive rats. Nat Genet 21: 76–83, 1999.
Hajri T, Han XX, Bonen A, et al: Defective fatty acid uptake modulates insulin responsiveness and metabolic responses to diet in CD36-null mice. J Clin Invest 109: 1381–1389, 2002.
Glazier AM, Scott J, Aitman TJ: Molecular basis of the CD36 chromosomal deletion underlying SHR defects in insulin action and fatty acid metabolism. Mamm Genome 13: 108–113, 2002.
Pravenec M, Landa V, Zidek V, et al: Transgenic rescue of defective CD36 ameliorates insulin resistance in spontaneously hypertensive rats. Nat Genet 27: 156–158, 2001.
Hajri T, Ibrahimi A, Coburn CT, et al: Defective fatty acid uptake in the spontaneously hypertensive rat is a primary determinant of altered glucose metabolism, hyperinsulinemia, and myocardial hypertrophy. J Biol Chem 276: 23661–23666, 2001.
Febbraio M, Abumrad NA, Hajjar DP, et al: A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J Biol Chem 274: 19055–19062, 1999.
Gotoda T, Iizuka Y, Kato N, et al: Absence of CD36 mutation in the original spontaneously hypertensive rats with insulin resistance. Nat Genet 22: 226–228, 1999.
Febbraio M, Podrez EA, Smith JD, et al: Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J Clin Invest 105: 1049–1056, 2000.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamashita, S., Hirano, KI., Kuwasako, T. et al. Physiological and pathological roles of a multi-ligand receptor CD36 in atherogenesis; insights from CD36-deficient patients. Mol Cell Biochem 299, 19–22 (2007). https://doi.org/10.1007/s11010-005-9031-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-005-9031-4