Abstract
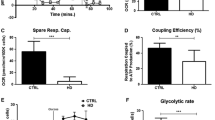
Huntington’s disease (HD) is associated with expansion of polyglutamine tract in a protein named huntingtin (htt) that is expressed in virtually all body tissues. Thus mutated htt (HD-htt) might affect all organs, although clinical manifestations of HD are associated with selective loss of corticostriatal neurons of the brain. In this work we studied how HD-htt affects mitochondria in human peripheral blood cells. We compared various functions of mitochondria isolated from cultured lymphoblastoid cells derived from three HD patients with juvenile onset of the disease (HD-LBM) and three age-matched control (C-LBM) individuals. Respiratory parameters in different metabolic states, with succinate and glutamate plus malate were the same for all control and HD cell lines. State 4 membrane potential in HD-LBM was slightly lower than in C-LBM. The calcium retention capacity (CRC) of mitochondria was estimated using simultaneously several methods to register permeability transition (PT). We found that LBM do not undergo swelling upon Ca2+-induced PT, and do not increase CRC in the presence of ADP + oligomycin. Although each cell line had different CRC values, qualitatively PT was different in C-LBM and HD-LBM. With C-LBM cyclosporin A (CsA) increased CRC significantly, while with HD-LBM CsA was ineffective. In C-LBM depolarization of mitochondria and a large pore opening (PT) always occurred simultaneously. In HD-LBM depolarization occurred at 20–50% lower Ca2+ loads than PT. We suggest that HD-htt promotes low H+ conductance of the mitochondria by interacting with proteins at the contacts sites without directly promoting PT or hampering mitochondrial oxidative phosphorylation. (Mol Cell Biochem 269: 143–152, 2005)
Similar content being viewed by others
References
Petersen A, Mani K, Brundin P: Recent advances on the pathogenesis of Huntington’s disease. Experim Neurology 157: 1–18, 1999
Rigamonti D, Nauer JH, De-Fraja C, Conti L, Sipione S, Sciorati C, Clementi E, Hackam A, Hayden MR, Li Y, Cooper JK, Ross CA, Govoni S, Vincenz C, Cattaneo E: Wild-type huntingtin protects from apoptosis upstream of caspase-3. J Neurosci 20: 3705–3713, 2000
Liu YF: Expression of polyglutamine-expanded Huntingtin activates the SEK1-JNK pathway and induces apoptosis in a hippocampal neuronal cell line. J Biol Chem 273: 28873–28877, 1998
Lear JD: Polyglutamine toxicity: A new idea for a tough problem. Biophys J 78: 2733–2734, 2000
Saudou F, Finkbeiner S, Devys D, Greenberg ME: Huntingtin acts in the nucleus to induce apoptosis but death does not correlate with the formation of intranuclear inclusions. Cell 95: 55–66, 1998
Cummings CJ, Zoghbi HY: Trinucleotide repeats: Mechanisms and pathophysiology. Ann Rev Hum Genet 1: 281–328, 2000
Schapira AH: Mitochondrial involvement in Parkinson’s disease, Huntington’s disease, hereditary spastic paraplegia and Friedreich’s ataxia. Biochim Biophys Acta 1410:159–170, 1999
Vonsattel JP, DiFiglia M: Huntington’s disease. J Neuropathol Exp Neurol 57: 369–384, 1998
Strong TV, Tagle DA, Valdes JM: Widespread expression of the human and rat Huntington’s disease gene in brain and non-neural tissues. Nat Genet 5: 259–265, 1993
Nicholls DG, Budd SL: Mitochondria and neuronal survival. Physiol Rev 80: 315–360, 2000
Bernardi P: Mitochondrial transport of cations: Channels, exchangers, and permeability transition. Physiol Rev 79: 1127–1155, 1999
Crompton M: The mitochondrial permeability transition pore and its role in cell death. Biochem J 341: 233–249, 1999
Petit PX, Susin S-A, Zamzami N, Mignotte B, Kroemer G: Mitochondria and programmed cell death: Back to the future. FEBS Letters 396: 7–13, 1996
Panov A, Obertone T, Bennett-Desmelik J, Greenamyre JT: Ca2+-dependent permeability transition and Complex I activity in lymphoblast mitochondria from normal individuals and patients with Huntington’s or Alzheimer’s disease. Ann NY Acad Sci 893: 365–368, 1999
Beutner G, Ruck A, Riede B, Brdiczka D: Complexes between porin, hexokinase, mitochondrial creatine kinase and adenylate translocator display properties of the permeability transition pore. Implication for regulation of permeability transition by the kinases. Biochim Biophys Acta 1368: 7–18, 1998
Shimizu S, Narita M, Tsujimoto Y: Bcl-2 family proteins regulate therelease of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature 399: 483–487, 1999
Panov A, Andreeva L, Greenamyre JT: Quantitative evaluation of the effects of mitochondrial permeability transition pore modifiers on accumulation of calcium phosphate: Comparison of rat liver and brain mitochondria. Arch Biochem Biophys,in press
Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, MacGregor GR, Wallace DC: The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature 427: 461–465, 2004
Kushnareva YuE, Campo ML, Kinnally KW, Sokolove PM: Signal presequences increase mitochondrial permeability and open multiple conductance channel. Arch Biochem Biophys 366: 107–115, 1999
Trounce IA, Kim YL, Jun AS, Wallace DC: Assessment of mitochondrial oxidative phosphorylation in patient muscle biopsies, lymphoblasts, and transmitochondrial cell lines. Methods Enzymol 264: 484–509, 1996
Sawa A, Wiegand GW, Cooper J, Margolis RL, Sharp AH, Lawler JF, Jr, Greenamyre JT, Snyder SH, Ross CA: Increased apoptosis of Huntington disease lymphoblasts associated with repeat length-dependent mitochondrial depolarization. Nature Medicine 5: 1194–1198, 1999
Panov AV, Gutekunst C-A, Leavitt BR, Hayden MR, Burke JR, Strittmatter WJ, Greenamyre JT: Early mitochondrial calcium defects in Huntington’s Disease area direct effect of polyglutamines. Nature Neurosci 5: 731–736, 2002
Wallace DC: Mitochondrial diseases in man and mouse. Science 283: 1482–1488, 1999
Brustovetsky N, Brustovetsky T, Jemmerson R, Dubinsky JM: Calcium-induced cytochrome c release from CNS mitochondria is associated with the permeability transition and rupture of the outer membrane. J Neurochem 80: 207–218, 2000
Shabalina IG, Panov AV: Control of energization of the liver mitochondria at the level of the adenine nucleotides carrier under condition of low tonicityof the incubation medium. Biochemistry (Moscow) 56: 282–287, 1991
Panov A, Filippova S, Lyakhovich V: Adenine nucleotide translocase as a site of regulation by ADP of the rat liver mitochondria permeability to H+ and K+ ions. Arch Biochem Biophys 199: 420–426, 1980
Panov A, Scarpa A: Mg2+ control of respiration in isolated rat liver mitochondria. Biochemistry 35: 12849–12856, 1996
LaNoue KF, Strzelecki T, Strzelecka D, Koch C: Regulation of the uncoupling protein in brown adipose tissue. J Biol Chem 261: 298–305, 1986
Halestrap AP, McGivan JD: Measurement of membrane transport phenomena. In: H.L. Kornberg, J.C. Metcalfe, D.H. Northcote, C.I. Pogson, K.F. Tipton (eds). Techniques in Meta bolic Research. Elsevier/North-Holland, Amsterdam, 1989, Vol. B206, pp 1–23
Tsien RY, Rink TJ: Neutral carrier ion-selective microelectrodes for measurement of intracellular free calcium. Biochim Biophys Acta 599: 623–638, 1980
Chalmers S, Nicholls DG: The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria. J Biol Chem 278: 19062–19070, 2003
Tabrizi SJ, Cleeter MWJ, Xuereb J, Taanman J-W, Cooper JM, Schapira AHV: Biochemical abnormalities and excitotoxicity in Huntington’s disease brain. Ann Neurol 45: 25–32, 1999
Tatton WG, Olanow CW: Apoptosis in neurodegenerative diseases: The role of mitochondria. Biochim Biophys Acta 1410: 195–213, 1999
Koroshetz WJ, Jenkins BG, Rosen BR, Beal MF: Energy metabolism defects in Huntington’s disease and effects of coenzyme Q. Ann Neurol 41: 160–165, 1997
Cortopassi GA, Wong A: Mitochondria in organismal aging and degeneration. Biochim Biophys Acta 1410: 183–193, 1999
Rego AC, Oliveira CR: Mitochondrial dysfunction and reactive oxygen species in excitotoxicity and apoptosis: Implications for the pathogenesis of neurodegenerative diseases. Neurochem Res 28: 1563–1574, 2003
Luthi-Carter R, Strand A, Peters NL, Solano SM, Hollingsworth ZR, Menon AS, Frey AS, Spektor BS, Penney EB, Schilling G, Ross CA, Borchelt DR, Tapscott SJ, Young AB, Cha JH, Olson JM: Decreased expression of striatal signaling genes in a mouse model of Huntington’s disease. Human Mol Genet 9: 1259–1271, 2000
Krohn, AJ, Wahlbrink T, Prehn JHM: Mitochondrial depolarization is not required for neuronal apoptosis. J Neurosci 19: 7394–7404, 1999
Savage MK, Reed DJ: Release of mitochondrial glutathione and calciumby a cyclosporin A-sensitive mechanism occurs without large amplitude swelling. Arch Biochem Biophys 315: 142–152, 1994
Mannella CA, Pfeiffer DR, Bradshaw PC, Moraru II, Slepchenko B, LoewLM, Hsieh C-E, Buttle K, Marko M: Topology of the mitochondrial inner membrane: Dynamics and bioenergetic implications. IUBMB Life 52: 93–100, 2001
Panov AV, Burke JR, Strittmatter WJ, Greenamyre JT: In vitro effectsof polyglutamine tracts on Ca2+-dependent depolarization of rat liver and human lymphoblast mitochondria: Relevance to Huntington’s disease. ArchBiochem Biophys 410: 1–6, 2003
Kushnareva YE, Sokolove PM: Prooxidants open both the mitochondrial permeability transition pore and a low-conductance channel in the inner mitochondrial membrane. Arch Biochem Biophys 376: 377–388, 2000
Nicholls DG, Ferguson SJ: Bioenergetics 3. Academic Press, London 2002
Monoi H, Futaki S, Kugimiya S-C, Minakata H, Yoshihara K: Poly-l-glutamine forms cation channels: Relevance to the pathogenesis of the polyglutamine diseases. Biophys J 78: 2892–2899, 2000
Norremolle A, Grunnet M, Hasholt L, Sorensen SA: Cells exposed to a huntingtin fragment containing an expanded polyglutamine tract show no sign of ion channel formation: Results arguing against the ion channel hypothesis. J Neurosci Res 71: 132–137, 2003
Wellington CL, Singaraja R, Ellerby L, Savill J, Roy S, Leavitt B, Cattaneo E, Hackam A, Sharp A, Thornberry N, Nicholson DW, Bredesen DE, Hayden MR: Inhibiting caspase cleavage of huntingtin reduces toxicity and aggregate formation in neuronal and non-neuronal cells. J Biol Chem 275: 19831–19838, 2000
Hansson MJ, Persson T, Friberg H, Keep MF, Rees A, Wieloch T, Elmer E: Powerful cyclosporin inhibition of calcium-induced permeability transition in brain mitochondria. Brain Res 960: 99–11, 2002
Schapira AHV: Mitochondrial function in Huntington’s disease: Clues for pathogenesis and prospects for treatment. Ann Neurol 41: 141–142, 1997
Nishino H, Hida H, Kumazaki M, Shimano Y, Nakajima K, Shimizu H, Ooiwa T, Baba H: The striatum is the most vulnerable region in the brain to mitochondrial energy compromise: A hypothesis to explain its specific vulnerability. J Neurotrauma 17(3): 251–260, 2000
Greene JG, Greenamyre JT: Bioenergetics and glutamate excitotoxicity. Progr Neurobiol 48: 613–634, 1996
Alexia T, Borlongand CV, Faullb RLM, Williamsa CE, Clarka RG, Gluckmana PD, Hughesc PE: Neuroprotective strategies for basal ganglia degeneration: Parkinson’s and Huntington’s diseases. Progr Neurobiol 60: 409–470, 2000
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Panov, A.V., Lund, S. & Greenamyre, J.T. Ca2+-induced permeability transition in human lymphoblastoid cell mitochondria from normal and Huntington’s disease individuals. Mol Cell Biochem 269, 143–152 (2005). https://doi.org/10.1007/s11010-005-3454-9
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11010-005-3454-9