Abstract
The clinical state of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been considered a pandemic disease (COVID-19) that is rapidly spreading worldwide. Despite all global efforts, the only treatment for COVID-19 is supportive care and there has been no efficient treatment to fight this plague. It is confirmed that patients with chronic diseases such as cardiovascular disorder and diabetes; are more vulnerable to COVID-19. In the severe type of COVID-19, laboratory findings showed a remarkably enhanced C-reactive protein, IL-6 serum, Iron, and ferritin, which suggest an inflammatory response. Inflammation results in iron homeostasis imbalance and causes iron overload, exacerbating the SARSCOV2 infection. More importantly, recent studies have established that SARS-CoV-2 needs iron for viral replication and also activation. As a result, managing iron overload in diabetic patients with COVID-19 could be an early therapeutic approach to limit the lethal inflammatory response of COVID-19. In this review, Deferoxamine (DFO) has been proposed as an effective iron chelator agent.
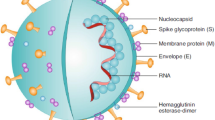
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Coronavirus disease (COVID-19) is caused by SARS-COV-2, which was introduced globally back in 2019. It has been recognized as multiple organ dysfunctions, which lead to death in severe cases (Zhou et al. 2020a, b). Following the first report in Wuhan in December 2019, the disease has rapidly spread, so it was eventually addressed as a pandemic by the World Health Organization (WHO) on Mar 11, 2020. The incidence of covid-19 has been reaching 660,131,952, of January 10, 2023.1, in which 6,690,473 cases of mortality have been recorded (Young et al. 2023). From the onset of the third wave, still, there is a threat of continuous rise with reports of new outbreaks of positive COVID-19 cases associated with the increased death toll, and despite vaccination on a large scale, in most countries, there is a need to prevent and eradicate different coronavirus variants (Mahajan et al. 2023).
The most common symptom clinical picture of COVID-19 includes fever, fatigue, dry cough, and shortness of breath. Patients may be asymptomatic or symptomatic varying from mild to severe infection. In some circumstances, it can cause pneumonia and multiple organ failure that eventually result in death. Evidently, patients with underlying diseases such as cardiovascular disorder and diabetes are more likely to confront the poor prognosis of COVID-19 (Holman et al. 2020; Kumar et al. 2020; Ruan et al. 2020).
Diabetes, as a lifelong condition, has been a cause for concern in the healthcare system all over the world. It is widely accepted that diabetes greatly impacts the innate immune system; as a result, the patients have a high predisposition to infection, compared to those without diabetes (Ardigo et al. 2004; Ferlita et al. 2019; Wu et al. 2020). To manage diabetes, diabetic patients require an adjusted diabetic treatment regimen when infected by the coronavirus (Wang et al. 2021). Studies have reported too many complications of COVID-19 such as Acute Respiratory Distress Syndrome (ARDS), cytokine storm, hypercoagulable state, destruction of hemoglobin, and iron overload resulting from dysregulated iron homeostasis. Liu et al. (2020) stated that the virus could cause iron overload by disassociating porphyrins from iron (Read 2020), leading to multi-organ failures due to a reduction in the oxygen-binding capacity of hemoglobin. Moreover, elevated free iron in the circulation produces excessive oxidative stress markers in several organs, which eventually cause inflammation and immune dysfunction (Moreira et al. 2020). Hence, iron-binding proteins uptake more iron, this may explain why ferritin levels increase in patients with COVID-19 (Zhou et al. 2020a, b). Furthermore, iron overload could induce deep venous thrombosis, and consequent pulmonary embolism accounted for sudden death in many cases (Gardenghi 2020).
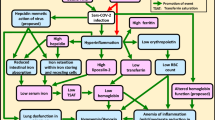
Iron overload and inflammation are proven to be the pathogenesis of diabetes (Fig. 1). On the other hand, this is well-documented that iron is crucial for replicating viral infection. For that reason, iron overload should be precisely managed in diabetic patients with COVID-19. Here, through investigating the existing studies, we aimed to determine whether deferoxamine as an iron chelator could alleviate COVID-19 complications in diabetic subjects by attenuating inflammation and hindering the coronavirus from entry into host cells.
Imbalance Iron Level as the Pathophysiology of Diabetes
Iron, an essential trace element in the human body, plays a pivotal role in immunity by activating macrophages during infection or inflammation. Macrophage activation is described as the first defense line against viral or bacterial attacks (Annous et al. 2021). In addition, iron is a crucial component of hemoglobin, the main oxygen carrier in the body (Dietz et al. 2021). A delicate balance between iron levels is necessary to provoke an immune response; hence the iron overload could be detrimental during inflammation and infection because viruses can take advantage of transferrin receptors to gain entry into cells (Chowdhury et al. 2021). This reveals why accumulated iron in cells during inflammation, which initially results from impaired iron homeostasis and iron deposition, leads to oxidative stress and cell death (Liu et al. 2019a, b). indisputably iron overload acts as one of the main pathologic pathways of diabetes. For instance, iron overload in the brain triggers inflammatory signaling pathways contributing to oxidative damage and cognitive decline (Liu et al. 2019a, b). Ferrous ion functions as a catalyst in the Fenton reaction and causes heightened oxygen toxicity by producing a wide range of free radical species such as aceruloplasminemia hydroxyl radicals (⋅OH) (Feng et al. 2020). Lately, the pathogenic role of iron in triggering inflammatory pathways in both type 1 and type 2 diabetes has been highlighted by several studies (Kataria et al. 2018). Several studies have introduced iron overload as one of the key clinical features of aceruloplasminemia in non-obese diabetic adults (Marchi et al. 2019). Iron chelators like Deferoxamine, Deferiprone, and Deferasirox can bind to excess iron to increase their urinary excretion. Different chelating agents exist in the liver to bind to excess hepatic iron and secrete them into bile. These chelators also absorb accumulated iron in the cardiomyocytes (Cui et al. 2015). Hosam et al. recently reported that lactoferrin (Lf), a naturally iron chelator, maintains immunomodulatory and anti-inflammatory effects. Consequently, it has bound to several coronaviruses' receptors and blocks their entry into host cells. This finding highlights the valuable function of iron chelators during the current pandemic (Habib et al. 2021; Mehta 2021). Although iron chelators have some complications, including ophthalmological and auditory toxicity, agranulocytosis, and neutropenia, their benefits outweigh the cost (Escolar et al. 2020).
Iron Overload: The Underlying Pathophysiology in Diabetic Patients with COVID-19
Diabetes is an inflammatory state associated with iron abnormalities and elevated oxidative stress markers (Van Campenhout, Van Campenhout et al. 2006; Zeinivand et al. 2020a, b). Several studies have recently confirmed that iron acts as a crucial pathogenic factor in type 1 and type 2 diabetes, which could be used as an efficient therapeutic target (Hansen et al. 2014). However, it is a paradoxical element since it is essential for living organisms and has toxic potential simultaneously (Heming et al. 2011). The fact that iron can reversibly oxidize is the mechanism of its pathophysiology. From one point of view, this tendency is critical for iron metabolic functions; but, also, it could lead to a hazardous process in which dangerous oxidant species like hydroxyl can be generated (Pouillevet et al. 2020). An abnormal hemoglobin level and related biochemical indices were reported in a study on 99 patients with COVID-19. The result showed that hemoglobin and neutrophil counts decreased in these patients.
Additionally, Indicator values of serum ferritin, erythrocyte sedimentation rate, C-reactive protein (CRP), albumin, and also lactate dehydrogenase were significantly reduced (Chen et al. 2020). Cells' response to stress usually produces high amounts of ferritin for binding to free iron and mitigating the possible damages. Lipinsky et al. showed a unique iron-related coagulation pathway in diabetic subjects, irrelevant to the common coagulation pathway which leads to proteolysis-resistant protein clotting accounting for triggering diabetic microangiopathy. They suggested prescribing chelating reagents like hydroxyl radical scavengers, including salicylates and iron polyphenols (Lipinski and Pretorius 2012). Ciciliano et al. reported a second mechanism of coagulation cascade activation caused by iron, in its configuration FeCl3 mediated through charge-based binding proteins. FeCl3 on the blood cells and the protein levels in the absence of endothelial cells proposed a charge-based mechanism. Insulin and glucose levels both appear to affect the coagulation cascade process (Ciciliano et al. 2015). Stregenga and colleagues reported a positive correlation between coagulation activation related to hyperglycemia and hyperinsulinemia impairing fibrinolysis. It could explain why high doses of insulin can inhibit pulmonary thrombosis (Banchini et al. 2020).
Furthermore, in a recent study, Haddadi et al. described a significant therapeutic effect of erythropoietin while treating an 80 year-old patient with COVID-19 and anemia. Supposedly, erythropoietin might have the ability to modulate iron distribution and metabolism. It has been assumed that COVID-19 infection may cause a significant effect on iron metabolism (Hadadi et al. 2020). Current evidence points to COVID-19 infection as a cause of iron overload via excessing intracellular iron, promoting pulmonary ferroptosis, mobilizing iron into the vascular space, and activating through an independent pathway of coagulation. Part of symptoms such as heart failure, profound asthenia, and fatigue might be directly associated with generating an excessive amount of reactive oxygen species (ROS) result from mitochondrial iron overload that eventually leads to more adverse consequences (Halon-Golabek et al. 2019; Edeas et al. 2020). More evidence has indicated that keeping balances between mitochondrial iron homeostasis and reducing iron content causes lower oxidative stress to prevent the formation of free radicals (Kumar et al. 2019; Abbasi et al. 2021).
Most importantly, it has been reported that when pro-inflammatory cytokines, such as IL-1α, IL-1β, and IL-6 are raised, hepcidin, a common regulator of iron homeostasis, increases, whereas ferroportin markedly down-regulates, result in hepcidin-mediated intracellular iron trapping (Habib et al. 2021). Inflammation-related rise of hepcidin probably induces internalizing cell surface ferroportin, so which disturbs its ability to export intracellular iron, causing it to store excess iron in the cells. Moreover, increasing hepcidin level further defects the iron import system by augmenting ferroportin’s degradation (Casu et al. 2018; Habib et al. 2021). Since SARS-CoV-2 requires iron for viral replication and activation, the role of iron chelation like deferoxamine should be particularly notified in diabetic patients whit COVID-19 (Liu et al. 2020).
Ferritin Levels in Patients with COVID-19
Ferritin, an essential intracellular source of iron storage, has two subunits, H and L. The proportion of these subunits is different based on tissue type and cells’ physiological state (Mesquita et al. 2020). Ferritin levels are raised due to inflammation, probably secreted by hepatocytes (Mesquita et al. 2020) and macrophages via a non-conventional pathway(Cohen et al. 2010), which highlighted the role of the macrophage in triggering ferritin production during hyperferritinemia syndromes. Ferritin has been described as a critical mediator of immune dysregulation, specifically during extreme hyperferritinemia, via direct immune-suppressive or pro-inflammatory effects, contributing to cytokine storm (Abbaspour et al. 2014; Ratiani et al. 2021). Cytokine storm, characterized by hyper inflammation, hyperferritinemia, and multi-organ failure resulting from excessive and uncontrolled cytokines production, stems from an exaggerated immune response, that claimed is one of the lethal outcomes of COVID-19 (Xu et al. 2020; Ombrello and Schulert 2021). Evidence has demonstrated that COVID-19 patients with ferritin levels higher than 300 μg/l, had a nine-fold increase in mortality and were more likely to present with severe symptoms than COVID-19 patients whose ferritin levels were much lower (Goldberg et al. 2020). There is extensive evidence that COVID-19 patients with elevated ferritin levels are more likely to develop more severe complications (Son 2019; Association 2020). Following previous results, we reviewed the evidence supporting the hypothesis that higher ferritin levels contribute to dramatically worsened side effects of COVID-19 (Vargas-Vargas and Cortés-Rojo 2020). A rise in ferritin levels has been confirmed among 20 patients with COVID-19, either in severe or very severe cases. However, in the very severe group, the ferritin level was significantly higher compared to the severe group. Not surprisingly, in those who died of COVID-19, ferritin level was elevated on the first day of admission or during hospitalization. The median ferritin concentration reached its upper limit on day 16 of hospitalization, indicating ferritin concentration was increasing continuously (Vargas-Vargas and Cortés-Rojo 2020). A descriptive study conducted in Wuhan, China, reported that 62 out of 99 (63%) patients clinically diagnosed with COVID-19 had serum ferritin higher than the normal range (Chen, Zhou et al. 2020a, b). High ferritin levels were also found in the autopsies of 12 patients who died of SARS-CoV-2 infection (Fox et al. 2020). Another analysis showed higher ferritin levels in 69 subjects with severe COVID-19 compared to those with non-severe diseases. As a result, presumably, the higher serum ferritin levels, the more severe complication of COVID-19 occurs. In this respect, a raised level of inflammatory markers related to cytokine storm, like ferritin, was shown in laboratory findings of patients with severe COVID-19 (Mehta et al. 2020; Mustafić et al. 2021).
Hyperferritinemia is largely considered an indicator of the “hyperferritinemia syndromes” associated with severe COVID-19 (Zandman-Goddard and Shoenfeld 2008; Edeas et al. 2020). Studies have demonstrated that high ferritin levels initiate acute and inflammatory responses (Colafrancesco et al. 2014; Edeas et al. 2020). The correlation between the severity of COVID-19 and high ferritin levels was also reported in a cohort study of 39 patients (Colafrancesco et al. 2020). Furthermore, a rise in ferritin could fatally harm hepatocytes during an inflammatory response because of remarkably high free iron levels (Pretorius and Kell 2014). Moreover, inflammatory conditions produce excessive free iron, which exacerbates the inflammatory reaction and increases the likelihood of coagulopathy conditions associated with poor prognosis (Pretorius and Kell 2014). The coronavirus, debatably, might show hepcidin-like effects that increase the risk of coagulopathy once again (Cavezzi et al. 2020).
After acute COVID-19 infection, some subjects develop a post-COVID-19 syndrome known as long-COVID. In these cases, researchers identified significant changes in proteins involved in iron metabolisms such as ceruloplasmin (Cp), transferrin (Tf), hemopexin (HPX), lipocalin 2 (LCN2), and superoxide dismutase 1 (SOD1). Moreover, activation of 5-lipoxygenase (5-LOX) in COVID-19 and in long-COVID have been reported, possibly developing through an iron-dependent post-translational mechanism (Dufrusine et al. 2023).
Hence iron chelators could also be beneficial to reduce ferritin levels. In this regard, deferoxamine appears to be the most suitable option as it is a non-toxic and FDA-approved iron chelator. Deferoxamine has been successfully administered for long-term iron chelation therapy in beta-thalassemia and other iron overload conditions (Ju and Ha 2016; Vargas-Vargas and Cortés-Rojo 2020). To sum up, reducing ferritin levels should be considered in COVID-19 patients, especially in elevated ferritin conditions like diabetes (Fan et al. 2019).
Iron Overload and Iron Chelators
Iron overload is characterized by increasing free and non-transferrin-bound iron (NTBI) in the plasma, resulting from tissue damage such as liver, lung, and heart (Kang et al. 2019). Cells’ iron toxicity occurs when Haber–Weiss and Fenton’s reactions are triggered by excessive free iron, generating a toxic hydroxyl radical (⋅OH) (Wongjaikam et al. 2016). In addition, cytotoxic malondialdehyde is also produced because of ROS production, resulting from plasma membrane lipid peroxidation (Aziza et al. 2014; Wongjaikam et al. 2016). Mitochondrial dysfunction and its consequent reduced cellular respiratory capacity arise from iron-catalyzed oxidants that cause mitochondrial DNA damage (Gao et al. 2010). Patients with iron overload are usually prescribed an iron chelator such as deferoxamine. Inhibiting Fenton reactions is the mechanism by which iron chelators can reduce free iron damage. By this, they prevent the production of hydroxyl radicals and ROS that extort oxidative damage and ferroptosis (Wang et al. 2019). Ferroptosis is a controlled cell death (Stockwell et al. 2017) related to raised free iron concurrent with ROS production (Alvarez et al. 2017; Kolnagou et al. 2018). Furthermore, iron chelators can (Cavezzi et al. 2020) perform anti-ferritin properties by hepcidin down-regulation, resulting in mitigating “hyperferritinemia syndromes” related to COVID-19 (Liu et al. 2020).
Increased ferritin production in the acute phase of SARS-CoV-2 infection might curb the pathogen replication, causing increased plasma non-transferrin bound iron (NTBI) that finally changes to labile plasma iron (LPI), the redox-active form (Perricone et al. 2020). This circumstance could be ended in tissue damage due to LPI elevated ferritin levels and ROS production (Le Lan et al. 2005). As a result, interrupting these steps with iron chelators like deferoxamine as an adjunct for effective drug targeting and new drugs development could be a dramatic approach to managing COVID-19 infection (Mahajan et al. 2023).
Deferoxamine can trigger autophagy in lysosomes so that it directly targets ferritin degradation. Deferoxamine converts the water-insoluble stored iron (ferritin) to a water-soluble form (transferrin); thereby, excess iron can be excreted into the urine. Although deferoxamine is a lipophilic molecule with a limited ability to chelate intracellular iron. After a few hours, it also reduces NTBI (Perricone et al. 2020).
Iron in COVID-19
Coronavirus (SARS-CoV-2) infection is also associated with inflammatory cytokine storm (Mehta et al. 2020), which consequently affects iron homeostasis (Weiss et al. 2019; Stebbing et al. 2020). Hepcidin, a novel iron regulator, controls gastrointestinal iron absorption and iron release. Inflammatory and infectious conditions give rise to hepcidin overexpression (Fillebeen et al. 2018; Zeinivand et al. 2020a, b; Zeinivand and Zavvari 2022). Coronavirus could cause hemolysis, so iron is released into circulation from porphyrins due to hemoglobin destruction. In consequence, ferritin production increases to reimburse the elevated free iron. High ferritin levels are detrimental for hepatocytes, so the loss of hepatocytes once again leads to a rise in free iron. This condition ends up exaggerated inflammatory response, elevated oxidative stress markers, and ferroptosis. Ferroptosis may elucidate why the COVID- related pulmonary injury does not directly derive from lung cell damage. Ferroptosis is a complex course of action that results in multiple organ failure and reduced lung capacity (Fillebeen et al. 2018). Moreover, Hypercoagulation was also observed in COVID-19 cases in which hydroxyl radical production cause fibrinogen to convert to fibrin clots which again attributed to excessive free iron. Interestingly, since serum iron status is lower in blood group O, there has been a relatively lower risk of COVID-19 among them (Fillebeen et al. 2018). Hence iron overload is a potential risk factor for COVID-19, and the effectiveness of iron chelators should also be noticed in treatment.
Iron Levels in Diabetic Patients with COVID-19
Iron is a crucial pathogenic element in type 1 and type 2 diabetes adjusting inflammatory responses (Hansen et al. 2014). Iron levels have been shown to surge up to 50% in diabetic subjects compared to non-diabetic controls (Prá et al. 2012). Ferritin, an acute-phase protein, augments during inflammation (Lee et al. 2011). There is an association between inflammation and Fe stores in diabetes. Indeed insulin resistant syndrome is characterized by elevated ferritin levels (Andrews et al. 2015). Besides diabetes, obesity, age and hypertension are defined as the risk factors for severe COVID-19 infection. In this sense, iron and hepcidin should be targeted when COVID-19 is concurrent with diabetes (Banchini et al. 2020). Bataille (Bataille et al. 2020) reported that higher ferritin levels are directly linked to the infection even when any symptoms are not observed, and the CRP result is negative, proposing ferritin levels as a manageable COVID-19 screening factor. In consistence, Connelly (Connelly et al. 1997) has submitted elevated ferritin levels as a predictor of ARDS, which is the most dangerous outcome of COVID-19. Not surprisingly, Dixon (Dixon et al. 2012) described ARDS as “Ferroptosis happens,” Abbas (Abbas et al. 2020) suggested iron chelators add-on therapy for COVID-19 patients after investigating the ferroptosis as to COVID-19 pathophysiology. Probably coronavirus has a significant impact on iron via the hepcidin pathway. Since iron overload and hepcidin overexpression might perform a critical role in diabetic patients with COVID-19, they must become the potential targets of the treatment strategy.
COVID-19 and Deferoxamine
Refractory hypoxemia defines acute respiratory distress syndrome (ARDS) related to Covid-19, and bilateral pulmonary infiltrates, associated with increased permeability of alveolar-capillary membranes. In addition, patients with ARDS are also subjected to severe oxidative stress caused by environmental stressors. Oxidative stress directly contributes to initiating and maintaining pathologic mechanisms by which respiratory viral infections violate homeostatic reactions. It is crucial to prevent systemic inflammation, and cytokine storms resulting from excessive reactive oxygen species. Additionally, ROS overproduction can negatively affect the expression of cell signaling proteins (Ciciliano et al. 2015). In other words, ROS-activated signaling pathways could change cell signaling proteins, combined whit a cytokine storm due to SARS-CoV2 infection, eventually giving rise to lung injury (Meftahi et al. 2021). The crucial role of iron overload in developing viral infections has been discussed for a long time. Iron can further enhance pro-oxidant reactions and trigger oxidative stress as the main physiopathology in lung diseases. In bronchoalveolar lavage fluids (BALF) of patients with ARDS, elevated iron level has been reported compared to normal healthy controls (Darenskaya et al. 2021; Meftahi et al. 2021). Signs and symptoms of life-threatening cytokine storm have been recorded among patients with severe COVID-19 (Chen, Zhou et al. 2020a, b). Hence, to decrease the mortality rate in COVID-19 patients, it has been highly recommended to identify and treat hyperinflammation (Mehta et al. 2020). For instance, Tocilizumab, a monoclonal antibody against interleukin-6 receptor, administration in patients with COVID-19 pneumonia and raised IL-6 has been approved (Mehta et al. 2020). An elevated plasma IL1B, IL1RA, IL7, IL8, IL9, IL10, basic FGF, GMCSF, IFNγ, IP10, MCP1, MIP1A, MIP1B, PDGF, TNFα, and VEGF has been found in patients with SARS-COV-2. Indeed, patients who had higher plasma levels of IL2, IL7, IL10, GCSF, IP10, MCP-1, MIP1A, and TNFα were classified as critically ill patients when admitted to intensive care units (ICU). These findings point out hyper-inflammatory conditions defined as cytokine storms (Chen, Zhou et al. 2020a, b). Moreover, to anticipate the severity and prognosis of adult COVID-19, detecting IL-6 parallel to D-Dimer concentrations has been proposed (Fu et al. 2020; Gao et al. 2020). The virus causes iron dysmetabolism, ferroptosis, and oxidative stress that adversely affect the host immune response (Schmidt 2020). An increased level of ferritin and iron in patients with COVID-19 is defined as an adverse prognostic factor that lethally interferes with endothelium, resulting in coagulation, and multiple organ dysfunction syndromes. Interestingly, iron chelators have been proven to abate viral replication and suppress consequent inflammatory pathways (Liu et al. 2020). In this review, we collectively hypothesized that as a safe and potent iron chelator, deferoxamine could attenuate complications related to iron overload in diabetic patients with COVID-19, regarding the maximum efficacy in managing other viral infections (Liu et al. 2020).
Conclusion
This review has developed the idea that deferoxamine may be useful in reducing iron overload-related inflammatory responses in diabetic patients with COVID-19. Coronaviruses are RNA viruses that are highly dependent on iron. Iron chelators like deferoxamine could be administered for COVID-19 cases considering their optimum efficacy in managing other viral infections (Liu et al. 2020). The virus causes iron dysmetabolism, ferroptosis, and oxidative stress that adversely affect the host immune response (Schmidt 2020). An increased level of ferritin and iron in patients with COVID-19 is defined as an adverse prognostic factor that lethally interferes with endothelium, coagulation, and multiple organ dysfunction syndromes. Interestingly, iron chelators have been proven to abate viral replication and suppress consequent inflammatory pathways (King et al. 2016; Liu et al. 2020).
As mentioned earlier, all outcomes indicate that diabetic patients are more likely to be affected by SARS-CoV-2. The present study has focused on the efficacy of deferoxamine as an anti-inflammatory and antioxidant agent (Mondal and Mukherjee 2020). More importantly, deferoxamine prevents pro-inflammatory cytokines (IL-1b, IL-6, and IL-8) secretion in the diabetic subject while increasing anti-inflammatory cytokines (IL-4 and IL-10). It also can decrease ferritin, and iron overload in a type 1 diabetes model and significantly reduced TNFα and IL-6 mRNA expression in diabetic subjects (Carboni et al. 2020; Park et al. 2020; Zeinivand et al. 2020a, b). It has been proposed that iron chelation as a beneficial adjuvant could treat COVID-19 patients (Lin et al. 2020; Liu et al. 2020). Lastly, more recent evidence has claimed that deferoxamine can manage COVID-19 infection (Dalamaga et al. 2020). The Covid-19 virus is more affected by iron imbalance compared to other viral infections. The complex interaction between iron, ferritin, cytokine storm, and inflammation must be investigated to clarify the precise mechanisms by which the COVID-19 virus leads to death. Measuring serum iron and ferritin levels could be considered as a marker to evaluate the severity of infection. Hence, reversing the iron overload by iron chelator could be a novel strategy to limit the fatal consequences of COVID-19 in diabetic patients.
It needs to mention that although studies show that the use of iron-chelators such as deferoxamine can be useful in reducing the infection of coronavirus, considering the key role of iron deficiency in anemia, or considering that iron itself is necessary for immune cell proliferation, and some evidence suggests that low iron increases the risk of some infection, more studies should be done to assess the optimal levels of iron that limit infection without posing a danger or side effect to the body such as iron deficiency anemia which may increase the risk of other illnesses (Menshawey et al. 2020).
References
Abbas A, Mostafa A, Yousof E, Ali S (2020) Use of iron chelators to reduce the severity of COVID-19. Thromb Haemost 4:1042
Abbasi U, Abbina S, Gill A, Takuechi LE, Kizhakkedathu JN (2021) Role of iron in the molecular pathogenesis of diseases and therapeutic opportunities. ACS Chem Biol. https://doi.org/10.1021/acschembio.1c00122
Abbaspour N, Hurrell R, Kelishadi R (2014) Review on iron and its importance for human health. J Res Med Sci 19(2):164
Alvarez SW, Sviderskiy VO, Terzi EM, Papagiannakopoulos T, Moreira AL, Adams S, Sabatini DM, Birsoy K, Possemato R (2017) NFS1 undergoes positive selection in lung tumours and protects cells from ferroptosis. Nature 551(7682):639–643
Andrews M, Soto N, Arredondo-Olguín M (2015) Association between ferritin and hepcidin levels and inflammatory status in patients with type 2 diabetes mellitus and obesity. Nutrition 31(1):51–57
Annous Y, Manning S, Khoujah D (2021) Ferritin, fever, and frequent visits: hyperferritinemic syndromes in the emergency department. Am J Emerg Med 48:249–254
Ardigo D, Valtuena S, Zavaroni I, Baroni MC, Delsignore R (2004) Pulmonary complications in diabetes mellitus: the role of glycemic control. Curr Drug Targ-Inflamm Allerg 3(4):455–458
Association, A. D. (2020). How COVID-19 Impacts People with Diabetes.
Aziza S, Mel-S A, El-Shall SK (2014) Ameliorating role of rutin on oxidative stress induced by iron overload in hepatic tissue of rats. Pak J Biol Sci: PJBS 17(8):964–977
Banchini F, Vallisa D, Maniscalco P, Capelli P (2020) Iron overload and Hepcidin overexpression could play a key role in COVID infection, and may explain vulnerability in elderly, diabetics, and obese patients. Acta Bio Medica: Atenei Parmensis 91(3):e2020013
Bataille S, Pedinielli N, Bergounioux J-P (2020) Could ferritin help the screening for COVID-19 in hemodialysis patients? Kidney Int 98(1):235–236
Carboni E, Carta AR, Carboni E (2020) Can pioglitazone be potentially useful therapeutically in treating patients with COVID-19? Med Hypotheses 140:109776
Casu C, Nemeth E, Rivella S (2018) Hepcidin agonists as therapeutic tools. Blood J Am Soc Hematol 131(16):1790–1794
Cavezzi A, Troiani E, Corrao S (2020) COVID-19: hemoglobin, iron, and hypoxia beyond inflammation. a narrative review. Clin Pract 10(2):24–30
Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet 395(10223):507–513
Chowdhury EA, Meno-Tetang G, Chang HY, Wu S, Huang HW, Jamier T, Chandran J, Shah DK (2021) Current progress and limitations of AAV mediated delivery of protein therapeutic genes and the importance of developing quantitative pharmacokinetic/pharmacodynamic (PK/PD) models. Adv Drug Deliv Rev. https://doi.org/10.1016/j.addr.2021.01.017
Ciciliano JC, Sakurai Y, Myers DR, Fay ME, Hechler B, Meeks S, Li R, Dixon JB, Lyon LA, Gachet C (2015) Resolving the multifaceted mechanisms of the ferric chloride thrombosis model using an interdisciplinary microfluidic approach. Blood J Am Soc Hematol 126(6):817–824
Cohen LA, Gutierrez L, Weiss A, Leichtmann-Bardoogo Y, Zhang D-L, Crooks DR, Sougrat R, Morgenstern A, Galy B, Hentze MW (2010) Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood J Am Soc Hematol 116(9):1574–1584
Colafrancesco S, Priori R, Alessandri C, Astorri E, Perricone C, Blank M, Agmon-Levin N, Shoenfeld Y, Valesini G (2014) sCD163 in AOSD: a biomarker for macrophage activation related to hyperferritinemia. Immunol Res 60(2–3):177–183
Colafrancesco S, Alessandri C, Conti F, Priori R (2020) COVID-19 gone bad: a new character in the spectrum of the hyperferritinemic syndrome? Autoimmun Rev 19(7):102573
Connelly KG, Moss M, Parsons PE, Moore EE, Moore FA, Giclas PC, Seligman PA, Repine JE (1997) Serum ferritin as a predictor of the acute respiratory distress syndrome. Am J Respir Crit Care Med 155(1):21–25
Cui H-J, He H-Y, Yang A-L, Zhou H-J, Wang C, Luo J-K, Lin Y, Tang T (2015) Efficacy of deferoxamine in animal models of intracerebral hemorrhage: a systematic review and stratified meta-analysis. PLoS ONE 10(5):e0127256
Dalamaga M, Karampela I, Mantzoros CS (2020) Commentary: Could iron chelators prove to be useful as an adjunct to COVID-19 treatment regimens? Metabolism 108:154260
Darenskaya M, Kolesnikova L, Kolesnikov S (2021) The association of respiratory viruses with oxidative stress and antioxidants. Implications for the COVID-19 pandemic. Curr Pharm Des 27(13):1618–1627
Dietz JV, Willoughby MM, Piel RB, Ross TA, Bohovych I, Addis HG, Fox JL, Lanzilotta WN, Dailey HA, Wohlschlegel JA (2021) Mitochondrial contact site and cristae organizing system (MICOS) machinery supports heme biosynthesis by enabling optimal performance of ferrochelatase. BioRxiv. https://doi.org/10.1016/j.redox.2021.102125
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149(5):1060–1072
Dufrusine B, Valentinuzzi S, Bibbò S, Damiani V, Lanuti P, Pieragostino D, Del Boccio P, D’Alessandro E, Rabottini A, Berghella A (2023) Iron dyshomeostasis in COVID-19: biomarkers reveal a functional link to 5-lipoxygenase activation. Int J Mol Sci 24(1):15
Edeas M, Saleh J, Peyssonnaux C (2020) Iron: innocent bystander or vicious culprit in COVID-19 pathogenesis? Int J Infect Dis 97:303–305
Escolar E, Ujueta F, Kim H, Mark DB, Boineau R, Nahin RL, Goertz C, Lee KL, Anstrom KJ, Lamas GA (2020) Possible differential benefits of edetate disodium in post-myocardial infarction patients with diabetes treated with different hypoglycemic strategies in the trial to assess chelation therapy (TACT). J Diabet Complicat 34(8):107616
Fan C, Song Q, Wang P, Li Y, Yang M, Yu SY (2019) Neuroprotective effects of curcumin on IL-1β-induced neuronal apoptosis and depression-like behaviors caused by chronic stress in rats. Front Cell Neurosci 12:516
Feng Y, Feng Q, Lv Y, Song X, Qu H, Chen Y (2020) The relationship between iron metabolism, stress hormones, and insulin resistance in gestational diabetes mellitus. Nutr Diabetes 10(1):1–6
Ferlita S, Yegiazaryan A, Noori N, Lal G, Nguyen T, To K, Venketaraman V (2019) Type 2 diabetes mellitus and altered immune system leading to susceptibility to pathogens, especially Mycobacterium tuberculosis. J Clin Med 8(12):2219
Fillebeen C, Wilkinson N, Charlebois E, Katsarou A, Wagner J, Pantopoulos K (2018) Hepcidin-mediated hypoferremic response to acute inflammation requires a threshold of Bmp6/Hjv/Smad signaling. Blood J Am Soc Hematol 132(17):1829–1841
Fox SE, Akmatbekov A, Harbert JL, Li G, Brown JQ, Vander Heide RS (2020) Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med 8(7):681–686
Fu Y, Cheng Y, Wu Y (2020) Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virologica Sinica 35(3):266–271
Gao X, Qian M, Campian JL, Marshall J, Zhou Z, Roberts AM, Kang YJ, Prabhu SD, Sun X-F, Eaton JW (2010) Mitochondrial dysfunction may explain the cardiomyopathy of chronic iron overload. Free Radical Biol Med 49(3):401–407
Gao Y, Li T, Han M, Li X, Wu D, Xu Y, Zhu Y, Liu Y, Wang X, Wang L (2020) Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol 92(7):791–796
Gardenghi G (2020) Pathophysiology of worsening lung function in COVID-19. Rev Brasileira De Fisiologia Do Exercí Cio 19(2):40–46
Goldberg MF, Goldberg MF, Cerejo R, Tayal A (2020) Cerebrovascular disease in COVID-19. Am J Neuroradiol 41(7):1170–1172
Habib HM, Ibrahim S, Zaim A, Ibrahim WH (2021) The role of iron in the pathogenesis of COVID-19 and possible treatment with lactoferrin and other iron chelators. Biomed Pharmacother. https://doi.org/10.1016/j.biopha.2021.111228
Hadadi A, Mortezazadeh M, Kolahdouzan K, Alavian G (2020) Does recombinant human erythropoietin administration in critically ill COVID-19 patients have miraculous therapeutic effects? J Med Virol 92(7):915–918
Halon-Golabek M, Borkowska A, Herman-Antosiewicz A, Antosiewicz J (2019) Iron metabolism of the skeletal muscle and neurodegeneration. Front Neurosci 13:165
Hansen J, Moen I, Mandrup-Poulsen T (2014) Iron: the hard player in diabetes pathophysiology. Acta Physiol 210(4):717–732
Heming N, Montravers P, Lasocki S (2011) Iron deficiency in critically ill patients: highlighting the role of hepcidin. Crit Care 15(2):1–7
Holman N, Knighton P, Kar P, O’Keefe J, Curley M, Weaver A, Barron E, Bakhai C, Khunti K, Wareham NJ (2020) Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol 8(10):823–833
Ju SY, Ha AW (2016) Dietary factors associated with high serum ferritin levels in postmenopausal women with the fifth Korea national health and nutrition examination survey (KNHANES V), 2010–2012. Nurs Res Pract 10(1):81–88
Kang H, Han M, Xue J, Baek Y, Chang J, Hu S, Nam H, Jo MJ, El Fakhri G, Hutchens MP (2019) Renal clearable nanochelators for iron overload therapy. Nat Commun 10(1):1–11
Kataria Y, Wu Y, Horskjær PDH, Mandrup-Poulsen T, Ellervik C (2018) Iron status and gestational diabetes—a meta-analysis. Nutrients 10(5):621
King GL, Park K, Li Q (2016) Selective insulin resistance and the development of cardiovascular diseases in diabetes: the 2015 Edwin Bierman Award Lecture. Diabetes 65(6):1462–1471
Kolnagou A, Kontoghiorghe CN, Kontoghiorghes GJ (2018) New targeted therapies and diagnostic methods for iron overload diseases. Front Biosci (schol Ed) 10:1–20
Kumar AA, Kelly DP, Chirinos JA (2019) Mitochondrial dysfunction in heart failure with preserved ejection fraction. Circulation 139(11):1435–1450
Kumar, A., A. Arora, P. Sharma, S. A. Anikhindi, N. Bansal, V. Singla, S. Khare, A. J. D. Srivastava, M. S. C. Research and Reviews (2020). "Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis." 14(4): 535-545
Le Lan C, Loréal O, Cohen T, Ropert M, Glickstein H, Lainé F, Pouchard M, Deugnier Y, Le Treut A, Breuer W (2005) Redox active plasma iron in C282Y/C282Y hemochromatosis. Blood 105(11):4527–4531
Lee B-K, Kim Y, Kim Y-I (2011) Association of serum ferritin with metabolic syndrome and diabetes mellitus in the South Korean general population according to the Korean national health and nutrition examination survey 2008. Metabolism 60(10):1416–1424
Lin Z, Long F, Yang Y, Chen X, Xu L, Yang M (2020) Serum ferritin as an independent risk factor for severity in COVID-19 patients. J Infect 81(4):647–679
Lipinski, B. and E. Pretorius (2012). "Novel pathway of iron‑induced blood coagulation: implications for diabetes mellitus and its complications."
Liu H, Hua Y, Keep RF, Xi G (2019a) Brain ceruloplasmin expression after experimental intracerebral hemorrhage and protection against iron-induced brain injury. Transl Stroke Res 10(1):112–119
Liu J, Wan M, Zhang Y, Zhang S, Zhang H, Wu S (2019b) Dysfunction of iron metabolism and iron-regulatory proteins in the rat hippocampus after heat stroke. Shock 51(6):780–786
Liu W, Zhang S, Nekhai S, Liu S (2020) Depriving iron supply to the virus represents a promising adjuvant therapeutic against viral survival. Curr Clin Microbiol Rep 7(2):13–19
Mahajan, S., M. Syed and S. Chougule (2023). "Microbial Iron Chelators: A Possible Adjuncts for Therapeutic Treatment of SARS-CoV-2 like Viruses."
Marchi G, Busti F, Lira Zidanes A, Castagna A, Girelli D (2019) Aceruloplasminemia: a severe neurodegenerative disorder deserving an early diagnosis. Front Neurosci 13:325
Meftahi G, Bahari Z, Jangravi Z, Iman M (2021) A vicious circle between oxidative stress and cytokine storm in acute respiratory distress syndrome pathogenesis at COVID-19 infection. Ukr Biochem J 93:18–29
Mehta KJ (2021) Role of iron and iron-related proteins in mesenchymal stem cells: cellular and clinical aspects. J Cell Physiol. https://doi.org/10.1002/jcp.30383
Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ (2020) COVID-19: consider cytokine storm syndromes and immunosuppression. The Lancet 395(10229):1033–1034
Menshawey R, Menshawey E, Alserr AH, Abdelmassih AF (2020) Low iron mitigates viral survival: insights from evolution, genetics, and pandemics—a review of current hypothesis. Egyptian J Med Hum Genet 21(1):1–14
Mesquita G, Silva T, Gomes AC, Oliveira PF, Alves MG, Fernandes R, Almeida AA, Moreira AC, Gomes MS (2020) H-Ferritin is essential for macrophages’ capacity to store or detoxify exogenously added iron. Sci Rep 10(1):1–15
Mondal K, Mukherjee D (2020) Study to assess association of C-reactive protein with nephropathy in people living with type 2 diabetes mellitus. MedRxiv. https://doi.org/10.1101/2020.09.23.20200519
Moreira AC, Mesquita G, Gomes MS (2020) Ferritin: an inflammatory player keeping iron at the core of pathogen-host interactions. Microorganisms 8(4):589
Mustafić, S., E. Jusufović, F. Hukić, E. Trnačević, A. Divković and A. Trnačević (2021). "Early predictors of severity and mortality in COVID-19 hospitalized patients." Med Glas (Zenica) 18 (2)
Ombrello, M. J. and G. S. Schulert (2021). "COVID-19 and cytokine storm syndrome: are there lessons from macrophage activation syndrome?" Translational Research.
Park S-M, Li Q, Ryu M-O, Nam A, An J-H, Yang J-I, Kim S-M, Song W-J, Youn H-Y (2020) Preconditioning of canine adipose tissue-derived mesenchymal stem cells with deferoxamine potentiates anti-inflammatory effects by directing/reprogramming M2 macrophage polarization. Vet Immunol Immunopathol 219:109973
Perricone, C., E. Bartoloni, R. Bursi, G. Cafaro, G. M. Guidelli, Y. Shoenfeld and R. Gerli (2020). "COVID-19 as part of the hyperferritinemic syndromes: the role of iron depletion therapy." Immunologic research: 1–12.
Pouillevet H, Soetart N, Boucher D, Wedlarski R, Jaillardon L (2020) Inflammatory and oxidative status in European captive black rhinoceroses: a link with iron overload disorder? PLoS ONE 15(8):e0231514
Prá D, Franke SIR, Henriques JAP, Fenech M (2012) Iron and genome stability: an update. Mutat Res/fundam Mol Mech Mutagenesis 733(1–2):92–99
Pretorius E, Kell DB (2014) Diagnostic morphology: biophysical indicators for iron-driven inflammatory diseases. Integr Biol 6(5):486–510
Ratiani L, Gabunia L, Khetsuriani S, Gamkrelidze N, Gogokhia N, Makharadze T, Varazi E, Antia N, Sulashvili N (2021) C reactive protein, procalcitonin, ferritin Levels in mild, severe and critically Ill patients with coronavirus disease 2019 (COVID-19). Int J Prog Sci Technol 26(2):281–286
Read, R. (2020). "Flawed methods in “COVID-19: attacks the 1-beta chain of hemoglobin and captures the porphyrin to inhibit human heme metabolism”."
Ruan Q, Yang K, Wang W, Jiang L, Song J (2020) Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 46(5):846–848
Schmidt SM (2020) The role of iron in viral infections. Front Biosci 25(4):893–911
Son NE (2019) Influence of ferritin levels and inflammatory markers on HbA1c in the Type 2 diabetes mellitus patients. Pak J Med Sci 35(4):1030
Stebbing J, Phelan A, Griffin I, Tucker C, Oechsle O, Smith D, Richardson P (2020) COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis 20(4):400–402
Stockwell BR, Angeli JPF, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK, Kagan VE (2017) Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171(2):273–285
Van Campenhout A, Van Campenhout C, Lagrou AR, Abrams P, Moorkens G, Van Gaal L, Manuel-y-Keenoy B (2006) Impact of diabetes mellitus on the relationships between iron-, inflammatory-and oxidative stress status. Diabet Metab Res Rev 22(6):444–454
Vargas-Vargas M, Cortés-Rojo C (2020) Ferritin levels and COVID-19. Rev Panam Salud Publica 44:e72
Wang B, Timilsena YP, Blanch E, Adhikari B (2019) Lactoferrin: structure, function, denaturation and digestion. Crit Rev Food Sci Nutr 59(4):580–596
Wang B, Glicksberg BS, Nadkarni GN, Vashishth D (2021) Evaluation and management of COVID-19-related severity in people with type 2 diabetes. BMJ Open Diabet Res Care 9(1):e002299
Weiss G, Ganz T, Goodnough LT (2019) Anemia of inflammation. Blood J Am Soc Hematol 133(1):40–50
Wongjaikam S, Kumfu S, Khamseekaew J, Sripetchwandee J, Srichairatanakool S, Fucharoen S, Chattipakorn SC, Chattipakorn N (2016) Combined iron chelator and antioxidant exerted greater efficacy on cardioprotection than monotherapy in iron-overloaded rats. PLoS ONE 11(7):e0159414
Wu H, Lau ES, Ma RC, Kong AP, Wild SH, Goggins W, Chow E, So W-Y, Chan JC, Luk AO (2020) Secular trends in all-cause and cause-specific mortality rates in people with diabetes in Hong Kong, 2001–2016: a retrospective cohort study. Diabetologia 63(4):757–766
Xu X, Chen P, Wang J, Feng J, Zhou H, Li X, Zhong W, Hao P (2020) Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci 63(3):457–460
Young Y, Shayya A, O’Grady T, Chen Y-M (2023) COVID-19 case and mortality rates lower in green houses compared to traditional nursing homes in New York State. Geriatr Nurs 50(2023):132–137
Zandman-Goddard G, Shoenfeld Y (2008) Hyperferritinemia in autoimmunity. Isr Med Assoc J 10(1):83
Zeinivand M, Zavvari F (2022) The beneficial role of Hepcidin peptide inhibitor in improved the symptoms of COVID-19 in diabetics: anti-inflammatory and potential therapeutic effects. J Diabet Metabol Disord. https://doi.org/10.1007/s40200-022-01053-9
Zeinivand M, Nahavandi A, Baluchnejadmojarad T, Roghani M, Golab F (2020a) Hepcidin peptide inhibitor as cardioprotection by targeting oxidative stress and inflammation in type 1 diabetic. Int J Pept Res Ther 26(2):1099–1106
Zeinivand M, Nahavandi A, Zare M (2020b) Deferoxamine regulates neuroinflammation and oxidative stress in rats with diabetes-induced cognitive dysfunction. Inflammopharmacology 28(2):575–583
Zhou C, Chen Y, Ji Y, He X, Xue D (2020a) Increased serum levels of hepcidin and ferritin are associated with severity of COVID-19. Med Sci Monit: Int Med J Exp Clin Res 26:e926171–e926178
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X (2020b) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet 395(10229):1054–1062
Acknowledgements
Present work was funded by a research grant from Kermanshah University of Medical Sciences, Kermanshah, Iran.
Author information
Authors and Affiliations
Contributions
MZ, MS, GH and SEN contributed to preparing manuscript and All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zeinivand, M., Sharifi, M., Hassanshahi, G. et al. Deferoxamine has the Potential to Improve the COVID-19-Related Inflammatory Response in Diabetic Patients. Int J Pept Res Ther 29, 63 (2023). https://doi.org/10.1007/s10989-023-10516-3
Accepted:
Published:
DOI: https://doi.org/10.1007/s10989-023-10516-3