Abstract
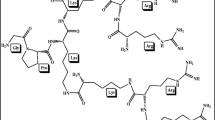
In inflammatory disorders (e.g. psoriasis), local concentrations of human neutrophil elastase (HNE), also known as polymorphonuclear leukocyte elastase (HLE), possibly overwhelm its natural inhibitors leading to extracellular matrix degradation. Elevated levels of HNE have been reported in a variety of inflammatory disorders, including psoriasis. Peptidic HNE inhibitors have a common hydrophobic sequence (Ala–Ala–Pro–Val). This peptide sequence inhibits HNE competitively; however the stratum corneum presents an effective barrier to the delivery of this tetrapeptide across the skin. The current work investigates the delivery of the modified peptide whereby the tetrapeptide was lipidated to enhance its ability to penetrate the stratum corneum. The tetrapeptide was coupled to a racaemic mixture of a short chain lipoamino acid (LAA) resulting in two diastereomers of the lipoamino acid-modified tetrapeptide. The penetration of the lipopeptide mixture was assessed across human epidermis in vitro. The percentage of applied dose penetrating to the receptor over 8 h following administration was 2.53% for the d-LAA conjugate and 1.47% for the l-diastereomer, compared to 0% for the peptide. The d-diastereomer appears to be relatively stable but the l-diastereomer appears to degrade releasing possibly the tetrapeptide and peptide fragment(s). Therefore the results clearly indicate that coupling the tetrapeptide to a short chain LAA enhances its delivery across human epidermis.




Similar content being viewed by others
Abbreviations
- Ala–Ala–Pro–Val:
-
l-alanyl–l-alanyl–l-prolyl–l-valine
- Boc:
-
t-butoxycarbonyl
- DCM:
-
dichloromethane
- DIEA:
-
diisopropylethylamine
- DMF:
-
dimethylformamide
- HBTU:
-
2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate
- HF:
-
hydrofluoric acid
- LAA:
-
lipoamino acid
- MBHA:
-
4-methylbenzhydrylamine
- Phe-Gly:
-
phenylalanyl-glycine
- TFA:
-
trifluoroacetic acid
References
Afouna M. I., Fincher T. K., Khan M. A., Reddy I. K., (2003) Chirality 15:456–465
Ahmed S., Imai T., Otagiri M., (1996) Pharm. Res. 13:1524–1529
Benson H. A. E., (2005) Curr. Drug Deliv. 2:23–33
Benson H. A. E., Caccetta R., Chen Y., Kearns P., Toth I., (2003) Lett. Pept. Sci. 10:615–620
Brittain H. G., (1990) Pharm. Res. 7:683–690
Cross S. E., Roberts M. S., (2004) Curr. Drug Deliv. 1:81–92
Gibbons, W. A., Hughs, A. S., Charalambous, M., Aulabaugh, A., Mascagni, P. and Toth I.: 1990, Liebigs Ann. Chem. 1175–1183
Hatanaka T., Suzuki R., Katayama K., Koizumi T., (1998) Int. J. Pharm. 168:199–208
Hornebeck W., Moczar E., Szecsi J., Robert L., (1985) Biochem. Pharmacol. 34:3315–3321
Kligman A., Christophers E., (1963) Arch. Dermatol. 88:702–705
Sarin V. K., Kent S. B. H., Tam J. P., Merrifield R. B., (1981) Anal. Biochem. 117:147–157
Schnölzer M., Alewood P., Jones A., Alewood D., Kent S. B. H. (1992) Int. J. Pept. Protein Res. 40:180–193
Thomas B. J., Finnin B. C., (2004) Drug Dis. Today 9:697–703
Toth I., Christodoulou M., Bankowsky K., Flinn N., Gibbons W. A., Godeau G., Moczar E., Hornebeck W., (1995) Int. J. Pharm. 125:117–122
Wiedow O., Wiese F., Streit V., Kalm C., Christophers E., (1992) J. Invest. Dermatol. 99:306–309
Yamamoto A., Setoh K., Murakami M., Shironoshita M., Kobayashi T., Fujimoto K., Okada N., Fujita T., Muranishi S., (2003) Int. J. Pharm. 250:119–128
Acknowledgments
This research was facilitated by a Curtin University of Technology Small Discovery/Linkage grant. The authors gratefully acknowledge the support of a number of Perth-based plastic surgeons and their patients for the donation of skin.
Author information
Authors and Affiliations
Corresponding author
Additional information
Australian Peptide Conference Issue.
Rights and permissions
About this article
Cite this article
Caccetta, R., Blanchfield, J.T., Harrison, J. et al. Epidermal Penetration of a Therapeutic Peptide by Lipid Conjugation; Stereo-Selective Peptide Availability of a Topical Diastereomeric Lipopeptide. Int J Pept Res Ther 12, 327–333 (2006). https://doi.org/10.1007/s10989-006-9024-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-006-9024-5