Abstract
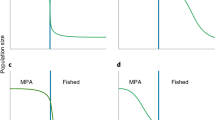
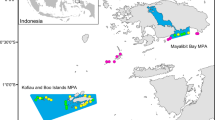
Source-sink theory has contributed to our understanding of the function of protected areas, particularly due to their role as population sources. Marine reserves are a preferred management tool for the conservation of natural populations, creating areas of good quality habitat and thus improving population connectivity by enhancing larval supply and recruitment among shores. Despite recent advances in the study of protected areas in the context of the source-sink theory, rigorous and empirical testing of marine reserves as metapopulation sources for the adjacent areas remain largely unexplored. We investigated the role of marine reserves as population sources, whether there was spill-over beyond the reserve boundaries and if so, whether spill-over was directional. We measured percentage cover and recruitment of mussels (Perna perna) at two reserves and two comparably sized exploited control areas on the south-east coast of South Africa where unprotected populations are severely affected by artisanal exploitation. Adult abundances were enhanced within reserves, but decreased towards their edges. We predicted that recruitment would mirror adult abundances and show directionality, with northern shores having greater recruitment following the prevalent northward flow of near-shore currents. There were, however, no correlations between adult abundances and recruitment for any months or shores, and no clear spatial patterns in recruitment (i.e. similar patterns occurred at reserves and controls). The results emphasise that, while reserves may act as important refuges by protecting adult abundances, their influence on promoting recovery of near-by exploited shores through larval spill-over may be overestimated.



Similar content being viewed by others
References
Airoldi L, Bacchiocchi F, Cagliola C, Bulleri F, Abbiati M (2005) Impact of recreational harvesting on assemblages in artificial rocky habitats. Mar Ecol Prog Ser 299:55–66
Berry PF (1978) Reproduction, growth and production in the mussel, Perna perna (Linnaeus), on the east coast of South Africa. Published by Oceanographic Research Institute. South African Association for Marine Biological Research Investigative Report, N. 48
Beukers-Stewart BD, Vause BJ, Mosley MWJ, Rossetti HL, Brand AR (2005) Benefits of closed area protection for a population of scallops. Mar Ecol Prog Ser 298:189–204
Bownes S, Barker NP, McQuaid CD (2008) Morphological identification of primary settlers and post-larvae of three mussel species from the coast of South Africa. Afr J Marine Sci 30:233–240
Branch GM, Odendaal F (2003) The effects of marine protected areas on the population dynamics of a South African limpet, Cymbula oculus, relative to the influence of wave action. Biol Conserv 114:255–269
Caley MJ, Carr MH, Hixon MA, Hughes TP, Jones GP, Menge BA (1996) Recruitment and the local dynamics of open marine populations. Ann Rev Ecol Syst 27:477–500
Cole VJ, McQuaid CD (2010) Bioengineers and their associated fauna respond differently to the effects of biogeography and upwelling. Ecology 91:3549–3562
Cole VJ, McQuaid CD, Nakin MDV (2011) Harvesting of bioengineers has cascading influences to infaunal assemblages. Biol Conserv 144:2088–2096
Connell JH, Keough MJ (1985) Disturbance and patch dynamics of subtidal marine animals on hard substrata. In: Pickett STA, White PS (eds) The ecology of natural disturbance and patch dynamics. Academic Press, New York, pp 125–151
Diffendorfer JE (1998) Testing models of source-sink dynamics and balanced dispersal. Oikos 81:417–433
Dye AH, Lasiak TA, Gabula S (1997) Recovery and recruitment of the brown mussel, Perna perna (L.) in Transkei: implications for management. S Afr J Zool 32:118–123
Erlandsson J, McQuaid CD (2004) Spatial structure of recruitment in the mussel Perna perna at local scales: effects of adults, algae and recruit size. Mar Ecol Prog Ser 267:173–185
Erlandsson J, Porri F, McQuaid CD (2008) Ontogenetic changes in small scale movement by recruits of an exploited mussel: implications for the fate of larvae settling on algae. Mar Biol 153:365–373
Fahrig L (2001) How much habitat is enough? Biol Conserv 100:65–74
Griffiths CL, Branch GM (1997) The exploitation of coastal invertebrates and seaweeds in South Africa: historical trends, ecological impacts and implications for management. Trans R Soc S Afr 52:121–148
Halpern BS (2003) The impact of marine reserves: Do reserves work and does reserve size matter? Ecol Appl 13:117–137
Hansen AJ (2011) Contribution of source-sink theory to protected area science. In: Liu J, Hull V, Morzillo A, Wiens JA (eds) Source, sinks, and sustainability across landscapes. Cambridge University Press, Cambridge, pp 339–360
Harris JM, Branch GM, Elliott BL, Currie B, Dye AH, McQuaid CD, Tomalin BJ, Velasquez C (1998) Spatial and temporal variability in recruitment of intertidal mussels around the coast of southern Africa. S Afr J Zool 33:1–11
Hockey PAR, Branch GM (1994) Conserving marine biodiversity on the African coast: implications of a terrestrial perspective. Aquat Conserv 4:345–362
Huggett AJ (2005) The concept and utility of ‘ecological thresholds’ in biodiversity conservation. Biol Conserv 124:301–310
Hunt HL, Scheibling RE (1996) Physical and biological factors influencing mussel (Mytilus trossulus, M. edulis) settlement on a wave exposed rocky shore. Mar Ecol Prog Ser 142:135–145
Kellner JB, Tetreault I, Gaines SD, Nisbet RM (2007) Fishing the line near marine reserves in single and multispecies fisheries. Ecol Appl 17:1039–1054
Kindlmann P, Burel F (2008) Connectivity measures: a review. Landscape Ecol 23:879–890
Lasiak TA, Barnard TCE (1995) Recruitment of the brown mussel Perna perna onto natural substrata: a refutation of the primary/secondary settlement hypothesis. Mar Ecol Prog Ser 120:147–153
Lasiak TA, Dye AH (1989) The ecology of the brown mussel Perna perna in Transkei, Southern Africa: implications for the management of a traditional food resource. Biol Conserv 47:245–257
Lasiak T, Field JG (1995) Community-level attributes of exploited and non-exploited rocky infratidal macrofaunal assemblages. J Exp Mar Biol Ecol 185:33–53
Leibold MA, Holyoak M, Mouquet MA, Amarasekare P, Chase JM, Hoopes F, Holt RD, Shurin JB, Law R, Tilman D, Loreau M, Gonzalez A (2004) The metacommunity concept: a framework for multi-scale community ecology. Ecol Lett 7:601–613
Lipcius RN, Stockhausen WT, Eggleston DB, Marshall LS Jr, Hickey BD (1997) Hydrodynamic decoupling of recruitment, habitat quality and adult abundance in the Caribbean spiny lobster: Source-sink dynamics? Mar Freshw Res 48:807–815
Lipcius RN, Eggleston DB, Schreiber SJ, Seitz RDS, L.C., Shen J, Sisson M, Stockhausen WT and Wang HV (2008) Importance of metapopulation connectivity to restocking and restoration of marine species. Rev Fish Sci 16:101–110
Manriquez PH, Castilla JC (2001) Significance of marine protected areas in central Chile as seeding grounds for the gastropod Concholepas concholepas. Mar Ecol Prog Ser 215:201–211
McQuaid CD, Lindsay JR (2005) Interacting effects of wave exposure, tidal height and substratum on spatial variation in densities mussel Perna perna plantigrades. Mar Ecol Prog Ser 301:173–184
McQuaid CD, Phillips TE (2000) Limited wind-driven dispersal of intertidal mussel larvae: in situ evidence from the plankton and the spread of the invasive species Mytilus galloprovincialis in South Africa. Mar Ecol Prog Ser 201:211–220
Meese RJ, Tomich PA (1992) Dots on the rocks: a comparison of percentage cover estimation methods. J Exp Mar Biol Ecol 165:59–73
Menge BA (1992) Community regulation: Under what conditions are bottom-up factors important on rocky shores? Ecology 73:755–765
Morgan SG (2001) The larval ecology of marine communities. In: Bertness MD, Gaines SD, Hay ME (eds) Marine community ecology. Sinauer Associates, Sunderland, pp 159–181
Naves J, Wiegand T, Revilla E, Delibes M (2003) Endangered species constrained by natural and human factors: the case of Brown Bears in Northern Spain. Conserv Biol 17:1276–1289
Paine RT (1966) Food web complexity and species diversity. Am Nat 100:65–75
Palumbi SR (2004) Marine reserves and ocean neighborhoods: the spatial scale of marine populations and their management. Ann Rev Environ Resour 29:31–68
Pelc RA, Baskett ML, Tanci T, Gaines S, Warner RR (2009) Quantifying larval export from South African marine reserves. Mar Ecol Prog Ser 394:65–78
Petraitis PS, Latham RE (1999) The importance of scale in testing the origins of alternative community states. Ecology 80:429–442
Pineda J, Reyns NB, Starczak VR (2009) Complexity and simplification in understanding recruitment in benthic populations. Popul Ecol 51:17–32
Porri F, McQuaid CD, Radloff S (2006) Spatio-temporal variability of larval abundance and settlement of Perna perna: differential delivery of mussels. Mar Ecol Prog Ser 315:141–150
Porri F, Zardi GI, McQuaid CD, Radloff S (2007) Tidal height, rather than habitat selection for conspecifics, controls settlement in mussels. Mar Biol 152:631–637
Pulliam RN (1988) Sources, sinks, and population regulation. Am Nat 138:652–661
Reaugh-Flower K, Branch GM, Harris JM, McQuaid CD, Currie B, Arthur D, Robertson B (2010) Patterns of mussel recruitment in southern Africa: a caution about using artificial substrata to approximate natural recruitment. Mar Biol 157:2177–2185
Reaugh-Flower K, Branch GM, Harris JM, McQuaid CD, Currie B, Dye A, Robertson B (2011) Scale-dependent patterns and processes of intertidal mussel recruitment around southern Africa. Mar Ecol Prog Ser 434:101–119
Roberts CM, Bohnsack JA, Gell F, Hawkins JP, Goodridge R (2001) Effects of marine reserves on adjacent fisheries. Science 294:1920–1923
Rowley R (1994) Marine reserves in fisheries management. Aquat Conserv 4:233–254
Sale PF, Cowen RK, Danilowicz BS, Jones GP, et al. (2005) Critical science gaps impede use of no take fishery reserves. Trends Ecol Evol 20:74–80
Seed R (1996) Patterns of biodiversity in the macroinvertebrate fauna associated with mussel patches on rocky shores. J Mar Biol Assoc UK 76:203–210
Siddall SE (1980) A clarification on the Genus Perna (Mytilidae). Bull Mar Sci 30(4):858–870
Siegfried WR, Hockey PAR, Crowe AA (1985) Exploitation and conservation of brown mussel stocks by coastal people of Transkei. J Appl Ecol 25:353–363
Sousa WP (1984) The role of disturbance in natural communities. Annu Rev Ecol Syst 15:353–391
Stephens EG, Bertness MD (1991) Mussel facilitation of barnacle survival in a sheltered bay habitat. J Exp Mar Biol Ecol 145:33–48
Tuck GN, Possingham HP (2000) Marine protected areas for spatially structured exploited stocks. Mar Ecol Prog Ser 192:89–101
Underwood AJ (1997) Experiments in ecology—their logical design and interpretation using analysis of variance. Cambridge University Press, Cambridge
Underwood AJ (1999) Physical disturbances and their direct effect on an indirect effect: responses of an intertidal assemblage to a severe storm. J Exp Mar Biol Ecol 232:125–140
Underwood AJ, Kennelly SJ (1990) Pilot studies for designs of surveys of human disturbance of intertidal habitats in New South Wales. Aust J Mar Freshw Res 41:165–173
Underwood AJ, Keough MJ (2001) Supply-side ecology—the nature of consequences of variations in recruitment of intertidal organisms. In: Bertness MD, Gaines SD, Hay ME (eds) Marine community ecology. Sinauer Associates, Sunderland, pp 183–200
Van Erkom Shurink C, Griffiths CL (1990) Marine mussels of southern Africa—their distribution patterns, standing stocks, exploitation and culture. J Shellfish Res 9:75–85
Williamson DH, Russ GR, Ayling AM (2004) No-take marine reserves increase abundance and biomass of reef fish on inshore fringing reefs of the Great Barrier Reef. Environ Conserv 31:149–159
Acknowledgments
The authors are grateful to two anonymous referees for improving an earlier version of the manuscript. The authors thank Eastern Cape Parks for permission to work within the reserves and the park rangers for assistance in finding sites. The authors thank also R. Mapukata, Z. Amos, M. Nkaitshana, C. von der Meden, L. Johnson, B. Mostert, M. Ludford, J. Booth, M. Goddard and Z. Gqamana for assistance and advice in the field. Funding was supplied by the Swedish International Development Cooperation Agency (SIDA) and the National Research Foundation of South Africa in a joint programme with the Swedish Research Council. This study is based upon research supported by the South African Research Chairs Initiative of the Department of Science and Technology and the National Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ludford, A., Cole, V.J., Porri, F. et al. Testing source-sink theory: the spill-over of mussel recruits beyond marine protected areas. Landscape Ecol 27, 859–868 (2012). https://doi.org/10.1007/s10980-012-9739-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-012-9739-y