Abstract
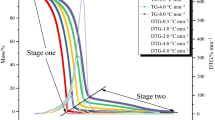
Michael addition reaction of acrylic acid has caused thermal runaway of acrylic acid, thereby causing many explosion and fire accidents in the chemical industries. The aim of the present study is to gain a better understanding of the reaction mechanisms of Michael addition oligomers of acrylic acid (MAO) during a runaway polymerization on the basis of thermal and evolved gas analysis. The Michael addition reaction of acrylic acid occurs at a temperature lower than the temperature at which thermal polymerization occurs, triggering the initiation of thermal polymerization and the associated thermal runaway, and after the thermal runaway, the pressure generated by the reaction with the product, MAO, can cause tank rupture. We discussed the reaction mechanisms of MAO including thermal polymerization and thermal decomposition on the basis of high-sensitivity reaction calorimetry and evolved gas analysis of MAO and its thermal polymer. From the results of the thermal analysis using Calvet-type calorimeter and thermogravimetry–differential scanning calorimetry-mass spectrometry-infrared spectrometry (TG-DSC-MS-IR), MAO can be polymerized exothermically at more than 180 °C, and its thermal polymer decomposes to propanoic acid, carbon dioxide, ethylene and low-molecular hydrocarbons such as methane, ethane, and cyclopropane at more than 195 °C. Michael addition oligomerization of acrylic acid was found to not only thermally initiate free radical polymerization due to its heat generation but also increase the possibility of the tank explosion after runaway polymerization due to the large pressure generation induced by thermal decomposition of MAO polymer.








Similar content being viewed by others
References
Center for Chemical Process Safety, CCPS. Guidelines for safe storage and handling of reactive materials. Amer Instit of Chem Eng. USA. 1995.
Levy L, Penrod J. The anatomy of an acrylic acid runaway polymerization. Plant/Operation Prog. 1989;8:105–8.
Nippon shokubai Co., Ltd. Himeji plant explosion and fire at acrylic acid production facility investigation report. Japan. 2013.
Kao C, Hu K. Acrylic reactor runaway and explosion accident analysis. J Loss Prev Proc Ind. 2002;15:213–22.
Kalfas G, Krieger T, Wilcox R. Improvements in the safety screening of resin manufacturing processes. Proc Safe Prog. 2009;28:275–81.
Gromacki M. Acrylic polymer reactor accident investigation: Lessons learned and three years later. CCPS International Conference and Workshop on Process Industry Incidents. USA. 2000.
Kurland J, Bryant D. Shipboard polymerization of acrylic acid. Plant/Operation Prog. 1987;6:203–7.
Gustin J. How the study of accident case histories can prevent runaway reaction accidents from recurring. Inst Chem Eng. 2002;80:16–24.
Krause G, Wehrstedt K, Malow M, Budde K, Mosler J. Safe transport of acrylic acid in railroad tank cars. Part 1: determination of the self-accelerating decomposition temperature. Chem Eng Technol. 2014;37:1460–7.
Fujita M, Iizuka Y, Miyake A. Thermal and kinetic analyses on Michael addition reaction of acrylic acid. J Therm Anal Calorim. 2017;128:1227–33.
Dubinsky S, Grader G, Shter G, Silverstein M. Thermal degradation of poly(acrylic acid) containing copper nitrate. Polym Degrad Stabil. 2004;86:171–8.
Maurer J, Eustace D, Ratcliffe C. Thermal characterization of poly(acrylic acid). Macromolecules. 1987;20:196–202.
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ. Gaussian 09, Revision C.01. Gaussian, Inc., Wallingford CT;2010.
Zhao Y, Truhlar D. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc. 2008;120:215–41.
Noble B, Coote M. First principles modelling of free-radical polymerisation kinetics. Int Rev Phys Chem. 2013;32:467–513.
Cuccato D, Dossi M, Moscatelli D, Storti G. Quantum chemical investigation of secondary reactions in poly (vinyl chloride) free-radical polymerization. Macromol React Eng. 2012;6:330–45.
Dossi M, Storti G, Moscatelli D. A quantum chemistry study of the free-radical copolymerization propagation kinetics of styrene and 2-hydroxyethyl acrylate. Polym Eng Sci. 2011;51:2109–14.
Dossi M, Liang K, Hutchinson R, Moscatelli D. Investigation of free-radical copolymerization propagation kinetics of vinyl acetate and methyl methacrylate. J Phys Chem B. 2010;114:4213–22.
Yu X, Pfaendtner J, Broadbelt L. Ab initio study of acrylate polymerization reactions: methyl methacrylate and methyl acrylate propagation. J Phys Chem A. 2008;112:6772–82.
Thickett S, Gilbert R. Propagation rate coefficient of acrylic acid: theoretical investigation of the solvent effect. Polymer. 2004;45:6993–9.
Zhang G, Konstantnov I, Arturo S, Yu D, Broadbelt L. Assessment of a cost-effective approach to the calculation of kinetic and thermodynamic properties of methyl methacrylate homopolymerization: a comprehensive theoretical study. J Chem Theory Comput. 2014;10:5668–76.
Montgomery J Jr, Frisch M, Ochterski J. A complete basis set model chemistry. VI. Use of density functional geometries and frequencies. J Chem Phys. 1999;110:2822–7.
Montgomery J Jr, Frisch M, Ochterski J. A complete basis set model chemistry. VII. Use of the minimum population localization method. J Chem Phys. 2000;112:6532–42.
Mennucci B, Cances E, Tomasi J. Evaluation of solvent effects in isotropic and anisotropic dielectrics and in ionic solutions with a unified integral equation method: theoretical bases, computational implementation, and numerical applications. J Phys Chem B. 1997;101:10506–17.
Marenich A, Cramer C, Truhlar D. Universal solvation model based on the generalized born approximation with asymmetric descreening. J Chem Theory Comput. 2009;5:2447–64.
Basic Acrylic Monomer Manufacturers Inc. Acrylic acid, a summary of safety and handling, 4th edn. USA: Basic Acrylic Monomer Manufacturers Inc.; 2013.
Alecu I, Zheng J, Zhao Y, Truhlar D. Computational thermochemistry: scale factor databases and scale factors for vibrational frequencies obtained from electronic model chemistries. J Chem Theory Comput. 2010;6:2872–87.
Evans A, Tyrrall E. Heats of polymerization of acrylic acid and derivatives. J Polym Sci. 1947;2:387–96.
Linstrom PJ, Mallard WG. NIST Chemistry WebBook. NIST standard reference database number 69. Eds. National Institute of Standards and Technology. http://webbook.nist.gov. Accessed 30 Jan 2020.
Izato Y, Koshi M, Miyake A, Habu H. Kinetics analysis of thermal decomposition of ammonium dinitramide (ADN). J Therm Anal Calorim. 2017;127:255–64.
Fujita M, Izato Y, Iizuka Y, Miyake A. Thermal hazard evaluation of runaway polymerization of acrylic acid. Process Saf Environ Protect. 2019;129:339–47.
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number JP 18KT0012, JP 20J15388 and the Environment Research and Technology Development Fund (JPMEERF20191004) of the Environmental Restoration and Conservation Agency of Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fujita, M., Izato, Yi. & Miyake, A. Thermal and evolved gas analyses on Michael addition oligomers of acrylic acid. J Therm Anal Calorim 147, 1825–1833 (2022). https://doi.org/10.1007/s10973-020-10412-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-10412-8