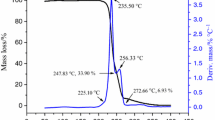
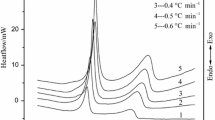
Abstract
The primary decomposition product (TKX-50-M) of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate (TKX-50) was obtained under adiabatic condition by using accelerating rate calorimeter (ARC). Meanwhile, diammonium 5,5′-bistetrazole-1,1′-diolate (ABTOX) was confirmed as the main component of TKX-50-M. Specific heat capacity of TKX-50-M and ABTOX was studied from 0 to 45 °C. In addition, the thermal decomposition of TKX-50-M and ABTOX was studied under adiabatic condition. The experiment results revealed that TKX-50-M were more thermal sensitive than ABTOX. Furthermore, ABTOX exhibited much violent than that of TKX-50-M during decomposition.








Similar content being viewed by others
References
Fischer N, Fischer D, Klapötke TM, Piercey DG, Stierstorfer J. Pushing the limits of energetic materials-the synthesis and characterization of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate. J Mater Chem. 2012;22:20418–22.
Fischer N, Klapötke TM, Reymann M, Stierstorfer J. Nitrogen-rich salts of 1H,1′H-5,5′-bitetrazole-1,1′-diol: energetic materials with high thermal stability. Eur J Inorg Chem. 2013;12:2167–80.
Klapötke TM, Witkowski TG, Wilk Z, Hadzik J. Determination of the initiating capability of detonators containing TKX-50, MAD-X1, PETNC, DAAF, RDX, HMX or PETN as a base charge, by underwater explosion test. Propellants Explos Pyrotech. 2016;41:92–7.
Gottfried JL, Klapötke TM, Witkowski TG. Estimated detonation velocities for TKX-50, MAD-X1, BDNAPM, BTNPM, TKX-55, and DAAF using the laser-induced air shock from energetic materials technique. Propellants Explos Pyrotech. 2017;42:1–8.
Xiong XL, Chen SS, Li LJ, Jin SH, Lin JL. Purity analysis method of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate (TKX-50). J Energy Mater. 2016;34:279–87.
Niu H, Chen SS, Jin SH, Shu QH, Li LJ, Shang FQ. Dissolution properties of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate and disodium 5,5′-bistetrazole-1,1′-diolate in water. J Energy Mater. 2016;34:416–25.
Yu YH, Chen SS, Li X, Zhu JP, Liang H, Zhang XX, Shu QH. Molecular dynamics simulations for 5,5′-bistetrazole-1,1′-diolate (TKX-50) and its PBXs. RSC Adv. 2016;6:20034–41.
Niu H, Chen SS, Jin SH, Li LJ, Shu QH. Dissolution thermodynamics of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate in water at T = (298.15, 303.15, 308.15 and 313.15 K). J Therm Anal Calorim. 2016;128:1–6.
Wang JF, Chen SS, Yao Q, Jin SH, Zhao SW, Yu ZF, Li JX, Shu QH. Preparation, characterization, thermal evaluation and sensitivities of TKX-50/GO composite. Propellants Explos Pyrotech. 2017;42:1104–10.
Niu H, Chen SS, Shu QH, Li LJ, Jin SH. Preparation, characterization and thermal risk evaluation of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate based polymer bonded explosive. J Hazard Mater. 2017;338:208–17.
Sinditskiia VP, Filatova SA, Kolesova VI, Kapranova KO, Asachenkob AF, Nechaevb MS, Luninb VV, Shishovc NI. Combustion behavior and physico-chemical properties of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate (TKX-50). Thermochim Acta. 2015;614:85–92.
Wang JF, Yang YF, Zhang CY, Wang XJ, Zhang XP. Thermal decomposition reaction kinetics of dihydroxylammonium-5,5′-bistetrazole-1,1′-diolate. Chin J Explos Propellants. 2015;38:42–5.
Huang HF, Shi YM, Yang J. Thermal characterization of the promising energetic material TKX-50. J Therm Anal Calorim. 2015;121:705–9.
Xiao LB, Zhao FQ, Luo Y, Li N, Gao HX, Xue YQ, Cui XZ, Hu HZ. Thermal behavior and safety of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate. J Therm Anal Calorim. 2016;123:653–7.
Muravyev NV, Monogarov KA, Asachenko AF, Nechaev MS, Ananyev IV, Fomenkov IV, Kiselevfg VG, Pivkina AN. Pursuing reliable thermal analysis techniques for energetic materials: decomposition kinetics and thermal stability of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate (TKX-50). Phys Chem Chem Phys. 2017;19:436–49.
Niu H, Chen SS, Jin SH, Li LJ, Jing BC, Jiang ZJ, Ji JW, Shu QH. Thermolysis, nonisothermal decomposition kinetics, calculated detonation velocity and safety assessment of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate. J Therm Anal Calorim. 2016;126:473–80.
Jia JH, Liu Y, Huang SL, Xu JJ, Li SC, Zhang HB, Cao X. Crystal structure transformation and step-by-step thermal decomposition behavior of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate. RSC Adv. 2017;7:49105–13.
Wang JF, Chen SS, Jin SH, Wang JY, Niu N, Zhang GY, Wang XY, Wang DX. Size-dependent effect on thermal decomposition and hazard assessment of TKX-50 under adiabatic condition. Propellants Explos Pyrotech. 2018;43:488–95.
Lesnikovich AI, Ivashkevich OA, Printsev GV, Gaponik PN, Levchik SV. Thermal decomposition of tetrazole Part III. Analysis of decomposition products. Thermochim Acta. 1990;171:207–13.
Lesnikovich AI, Ivashkevich OA, Levchik SV, Balabanovich AI, Gaponik PN, Kulak AA. Thermal decomposition of aminotetrazoles. Thermochim Acta. 2002;388:233–51.
Townsend DI, Tou JC. Thermal hazard evaluation by an accelerating rate calorimeter. Thermochim Acta. 1980;37:1–30.
Galwey AK, Mortimer M. Compensation effects and compensation defects in kinetic and mechanistic interpretations of heterogeneous chemical reactions. Int J Chem Kinet. 2006;38:464–743.
Barrie PJ. The mathematical origins of the kinetic compensation effect: 1 the effect of random experimental errors. Phys Chem Chem Phys. 2012;14:318–26.
Acknowledgements
This research was supported by “the Fundamental Research Funds for the Central Universities”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, J., Chen, S., Jin, S. et al. The primary decomposition product of TKX-50 under adiabatic condition and its thermal decomposition. J Therm Anal Calorim 134, 2049–2055 (2018). https://doi.org/10.1007/s10973-018-7820-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7820-8